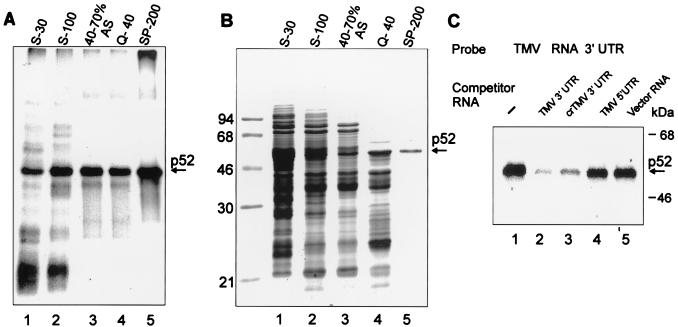
FIG. 3.
Purification of p52. (A) Specific UV cross-linking activity of protein fractions at different stages of p52 purification. Shown are proteins of crude wheat germ extract (S-30; 50 μg, lane 1) and selected fractions: S-100 postribosomal supernatant (S-100; 40 μg, lane 2), the 40 to 70% ammonium sulfate (AS) cut of S-100 (40 to 70% AS; 15 μg, lane 3), proteins from the 40 to 70% AS cut in the flowthrough from a Q-Sepharose column at 40 mM KCl (Q-40; 6 μg, lane 4), and protein from the Q-40 fraction eluted from a SP-Sepharose column with 200 mM KCl (SP-200; 1 μg, lane 5) were dialyzed to a salt concentration of 50 mM KCl before the assay (if required). Binding reactions, UV cross-linking to the 32P-labeled TMV U1 RNA 3′-UTR, RNase treatment, and SDS-PAGE were performed as described in Materials and Methods. (B) Protein composition of the selected fractions. Coomassie blue-stained SDS-12% PAGE of the same amounts of proteins from the fractions described for panel A. The SP-200 fraction contains one major protein band with a mobility of approximately 52 kDa as indicated. (C) UV cross-linking experiment with TMV U1 3′-UTR. The experiment was conducted as described for Fig. 2C, except that purified p52 was used and competitor RNAs were in 30-fold excess.