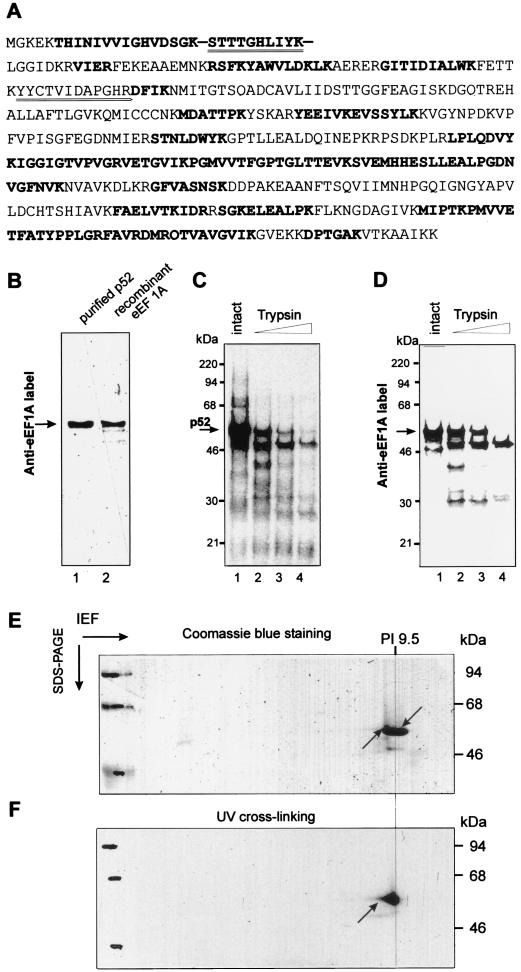
FIG. 4.
Identification of p52 as eEF1A. (A) Comparison of sequences of wheat germ eEF1A (Swissprot Q03033) and of peptides derived from p52. p52 from the SP-200 fraction was excised from the gel, digested with trypsin, and subjected to LC-MS as described in Materials and Methods. Peptides found by LC-MS are shown in bold. Additionally, two of the peptides were isolated and sequenced by Edman degradation (double underlined). (B) Identity of p52 and eEF1A as determined by Western blotting. Equal amounts (0.4 μg) of purified p52 and recombinant wheat eEF1A protein (see Materials and Methods) were fractionated on SDS-12% PAGE, transferred to nitrocellulose membrane, and incubated with rabbit polyclonal anti-wheat eEF1A antibody. Bound antibody was revealed by using an ECL kit (Amersham). (C and D) Comparative protease mapping of p52. Shown are proteins UV cross-linked to the 32P-labeled TMV U1 3′-UTR (C) and Anti-eEF1A activity in wheat germ extracts (S100) (C). (E and F) Two-dimensional isoelectric focusing/SDS-PAGE gel of purified p52. The purified p52 protein was UV cross-linked to 32P-labeled TMV U1 3′-UTR, treated with RNases, and resolved in the first dimension by NEPHGE and in the second dimension by SDS-PAGE. The gel was stained with Coomassie blue and analyzed by autoradiography as described in Materials and Methods. (E) Coomassie blue-stained gel. The two arrows indicate the positions of the Coomassie blue-stained and radiolabeled p52 spot. (F) Autoradiogram of the gel shown in panel E. The arrow indicates the position of the radiolabeled spot corresponding to p52.