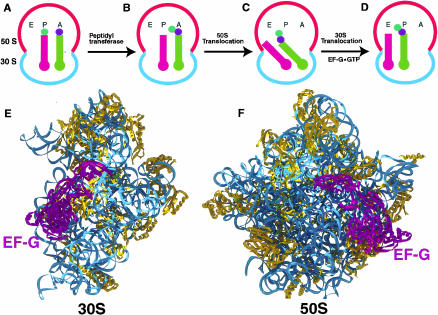
FIGURE 1.
Revised hybrid states model for translocation (Moazed and Noller 1989). (A) Ribosome with a peptidyl tRNA in the P/P state (stem–loop structure in magenta) and an aminoacyl tRNA in the A/A state (stem–loop structure in green). Colored circles represent an amino acid attached to the 3′-end of the tRNA. (B) After peptide-bond formation, deacylated tRNA is in the P/P state and peptidyl tRNA extended by one amino acid is in the A/A state (Schmeing et al. 2002). (C) The acceptor ends of the deacylated tRNA and the peptidyl tRNA are spontaneously translocated relative to the 50S subunit, resulting in P/E and A/P hybrid states, respectively. (D) EF-G moves the anticodon ends of the deacylated tRNA and peptidyl tRNA relative to the 30S subunit, resulting in E/E and P/P states, respectively. (E,F) Approximate position of EF-G relative to the 30S (Wimberly et al. 2000) and 50S (Ban et al. 2000) crystal structures viewed from the interface side. The docking of the EF-G crystal structure (AEvarsson et al. 1994) on the ribosomal subunits is based on data from directed hydroxyl radical probing (Wilson and Noller 1998a) and cryo-EM (Agrawal et al. 1998) studies.