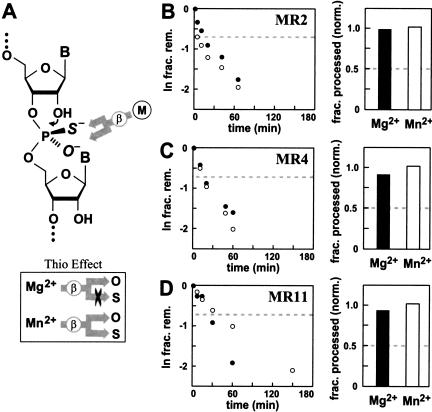
FIGURE 4.
Putative αγ ribozymes do not exhibit metal-mediated β catalysis. (A) Schematic representation of an Sp thiophosphate RNA linkage and the possible contacts made by a metal-ion cofactor during β catalysis. The sulfur atom will be located at either of the two nonbridging positions (Rp or Sp) with near equal distribution. If the enzyme is an obligate metalloenzyme for β catalysis, then the metal ion (M) is expected to coordinate selectively with only one nonbridging position, as established by the active site. The inset reflects the fact that Mn2+ can interact with oxygen or sulfur with similar affinity, whereas Mg2+ has high affinity only for oxygen. (B–D, left) Kinetic characteristics of MR2, MR4, and MR11 ribozymes with thiophosphate substrates in the presence of Mg2+ (filled circles) and Mn2+ (open circles). The dashed line designates the plateau in RNA cleavage that would be expected if a thio effect precluded the processing of one of the two isomers. (Right) The bar graph represents the fraction of total RNA substrate processed in the presence of Mg2+ or Mn2+. The fraction cleaved was corrected and normalized as detailed in Materials and Methods. Again, the dashed line designates the maximum value for RNA cleavage that is expected if Mg2+ could only process one of the two thiophosphate isomers.