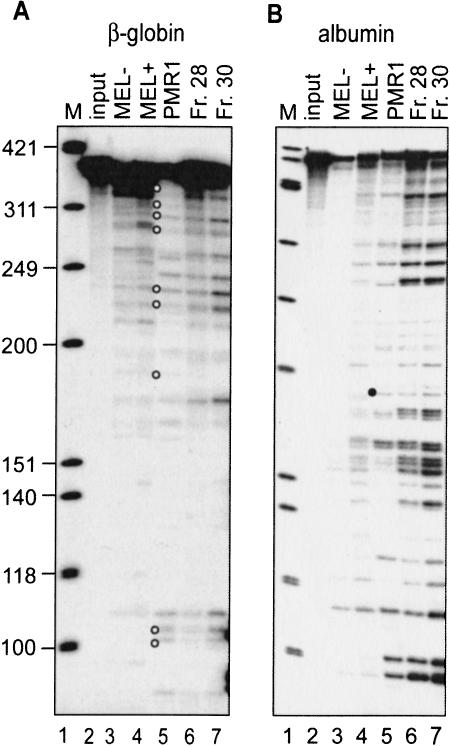
FIGURE 1.
In vitro cleavage of β-globin and albumin mRNA by PMR1 and the β-globin mRNA endonuclease. (A) A 5′ 32P-labeled transcript of the 5′-most 347 nucleotides of human β-globin mRNA was incubated for 30 min at 37°C with no added protein (lane 2, input), polysome extract from uninduced (MEL−, lane 3) or 48-h DMSO-induced MEL cells (MEL+, lane 4), or two fractions containing β-globin mRNA endonuclease activity recovered from Mono S fractionation of the polysome extract (lanes 6,7). Alternatively, the transcript was incubated for 20 min at 25°C with 20 U of purified Xenopus PMR1 (PMR1, lane 5). The reaction products were separated on a denaturing 6% polyacrylamide/urea gel and visualized by PhosphorImager. Lane 1 contains a marker consisting of φX174 DNA HinfI fragments and the open circles identify products with counterparts in vivo (Stevens et al. 2002). (B) An in vitro-synthesized, 5′ 32P-labeled transcript consisting of the 5′-most 420 nucleotide of Xenopus albumin mRNA was incubated as described in A. The characteristic PMR1 cleavage at overlapping APyrUGA elements is identified by a solid circle.