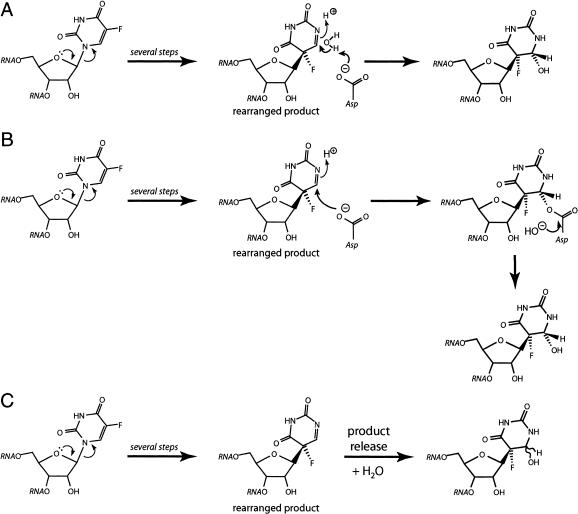
FIGURE 4.
Reaction routes that could explain the disparate behavior of Ψ synthases toward [f5U]RNA and the rearranged and hydrated product observed in the TruB•[f5U]TSL cocrystal structure (Hoang and Ferre-D’Amare 1991). In all three depicted schemes, the reaction can proceed by either proposed mechanism to generate the rearranged product. (A) The essential aspartic acid serves as a general base in the direct hydration of the rearranged product. (B) The essential aspartic acid residue performs a Michael addition at C6, followed by ester hydrolysis, which could be fast or slow; it is also possible that the ester forms as part of the mechanism involving Michel addition at C6, so that the depicted rearranged product never exists. (C) The rearranged product is released by the enzyme into solution and becomes hydrated, likely in a stereorandom manner.