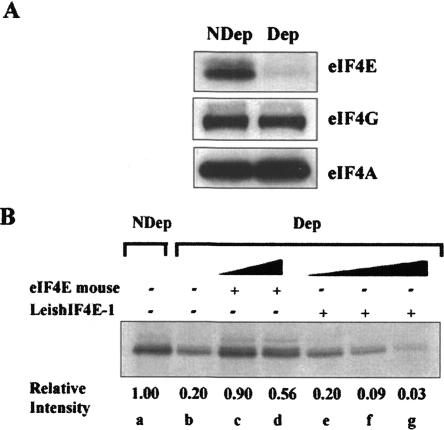
FIGURE 7.
LeishIF4E-1 cannot replace the mammalian eIF4E in an in vitro translation system. (A) eIF4E was selectively depleted from a rabbit reticulocyte system by addition of 4E-BP1, followed by m7GTP-Sepharose beads, which were used to remove the complex between eIF4E and the 4E-BP1. The selective depletion of eIF4E and not of other translation factors, was verified by Western analysis of the depleted (Dep) and nondepleted (NDep) extracts, using antibodies directed against the mammalian eIF4E, eIF4GI, and eIF4A. (B) Control nondepleted (NDep, lane a) lysates were used for translation of a capped and polyadenylated GFP transcript, in the absence (lane b) and in the presence of recombinant mouse eIF4E (2 μM, lane c; 10 μM, lane d) or recombinant LeishIF4E-1 (2 μM, lane e; 10 μM, lane f; 150 μM, lane g). The relative incorporation of [35S]methionine, measured by PhosphorImaging and compared with the nondepleted control, is given below the autoradiogram.