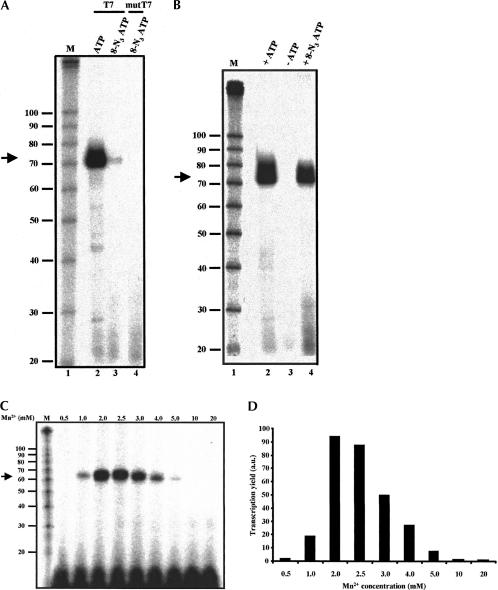
FIGURE 2.
8-N3ATP does not function as a substrate for T7 or mutant T7 (T7 R&DNA) polymerase under standard in vitro transcription conditions. (A) RNAs synthesized from a BamHI-digested plasmid pPIP85.B (Query et al. 1996), which directs the synthesis of 68-mer RNA, with indicated polymerase were resolved on a 10% polyacrylamide denaturing gel. A standard in vitro transcription reaction consists of 40 mM Tris-HCl, pH 8.0, 20 mM MgCl2, 2.0 mM spermidine, 5 mM DTT, 0.01% Triton X-100, 0.4 mM CTP, UTP, GTP, and ATP, ~5 μCi [α-32P-UTP], and 25–50 units RNA polymerase. In 8-N3ATP-containing transcription reactions, ATP and DTT were replaced with 0.4 mM 8-N3ATP and 5 mM β-mercaptoethanol, respectively. M, RNA size marker (Decade Marker, Ambion). (B) In the presence of Mn2+, , 8-N3ATP serves as a substrate for T7 RNA polymerase. As in panel A, except that Mg2+ was replaced with 2.5 mM Mn2+. (C) Effect of variation of metal ion concentration on T7 RNA polymerase-catalyzed synthesis of 8-N3AMP containing RNA. As described in A, transcription reactions were performed in the presence of different concentrations of Mn2+ (0.5–20 mM), and the products of transcription reactions were resolved on a 10% polyacrylamide denaturing gel. (D) Histogram representing the yield of RNA (panel C) as a function of Mn2+ concentration. a.u., arbitrary unit.