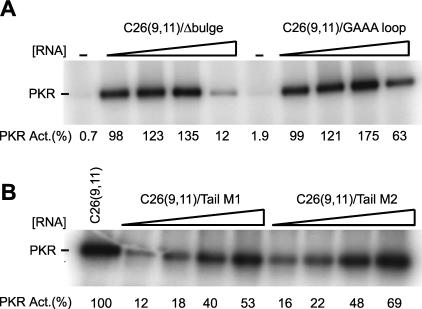
FIGURE 5.
Effects of deleting the bulge and varying the hairpin loop and 5′- and 3′-tails on PKR phosphorylation activity. In vitro activation assays of PKR on denaturing 10% SDS-polyacrylamide gels are shown. The position of phosphorylated PKR is noted. (A) Effects of bulge deletion and hairpin loop sequence on PKR activity. C26(9,11)/Δ bulge is a C-bulge-deleted C26(9,11), and C26(9,11)/ GAAA loop is a loop mutant of C26(9,11) in which the UGAAAUC loop was replaced with CGAAAG. Concentrations of RNAs were 0, 0.1, 0.3, 1.0, and 3.0 μM. Normalized phosphorylation activities are presented under the gel, and were normalized relative to the maximum in a C26(9,11) activity assay, which was run under identical electrophoresis and exposure conditions and occurred at 0.3–1.0 μM C26(9,11) (not shown). Deleting the bulge or changing the loop resulted in slightly elevated phosphorylation activities. (B) Effects of 5′-and 3′-flanking sequence on PKR activity. In both cases, the stem and loop were unchanged and the 5′- and 3′-tails were 9 and 11 nt, respectively. For C26(9,11)/Tail M1, most G’s in the 5′- and 3′-tails were changed to C’s, while for C26(9,11)/Tail M2, most G’s were changed to C’s, and all A’s were changed to U’s. (See Materials and Methods for exact sequences.) Concentrations of these RNAs were 0.1, 0.3, 1.0, and 2.0 μM. Normalized phosphorylation activities are presented under the gel and were normalized relative to 0.3 μM C26(9,11) (left-most lane), which is near concentration of C26(9,11) that has maximal activity (0.3–1.0 μM, depending on the experiment). Changing the sequence of the tails had only a minimal effect on phosphory-lation activity.