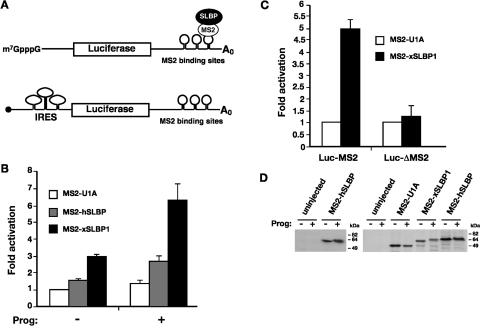
FIGURE 1.
SLBP activity increases during oocyte maturation. (A) Cartoon illustrating the tethered function assay. Luciferase reporter mRNAs contain binding sites for the MS2 coat protein within its 3′ UTR (Luc-MS2); SLBP is expressed as a fusion to MS2 coat protein. Binding of the coat protein tethers SLBP to the mRNA. The luciferase reporters have an m7GpppG (m7G) cap, an ApppG (ApG, black circle) cap, or contain a viral internal ribosome entry site (IRES) in their 5′UTRs, as indicated. An m7G-capped polyadenylated β-galactosidase mRNA (β-Gal) that contains no MS2 sites is utilized as an internal control. (B) In vitro transcribed m7G-capped Luc-MS2 and β-Gal mRNAs were co-injected into the cytoplasm of Xenopus stage VI oocytes expressing MS2-U1A, MS2-hSLBP, or MS2-xSLBP1. After micro-injection, half of the oocytes were incubated overnight in media containing 10 μg/mL of progesterone (prog). Luciferase activity was normalized to β-galactosidase activity and the fold activation relative to MS2-U1A (– progesterone) was plotted, with MS2-U1A values set to one. The average of at least four independent experiments is shown. (C) Stage VI oocytes expressing MS2-U1A or MS2-xSLBP1 were co-injected with m7G-capped Luc-MS2 or a luciferase reporter lacking MS2-binding sites (Luc-ΔMS2) and β-Gal mRNAs. Subsequently, oocytes were incubated overnight in media containing 10 μg/mL of progesterone. The average of three independent experiments is shown. (D) In vitro transcribed MS2-U1A, MS2-xSLBP1, or MS2-hSLBP were micro-injected into stage VI oocytes that were incubated overnight in the absence (−) or presence (+) of 10 μg/mL of progesterone. Oocytes were analyzed by Western blotting using anti-hSLBP antibody (left panel) or were labeled overnight with 50 μCi/mL 35S-methionine and immunoprecipitated with anti-MS2 antibody (right panel). Autoradiography of SDS-PAGE after fluorography is shown.