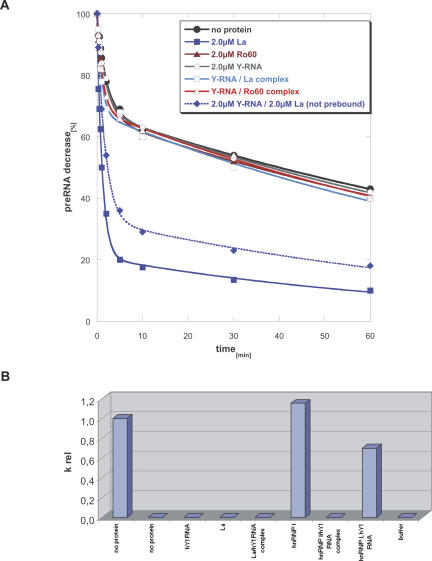
FIGURE 4.
Effect of Y RNA binding to individual components of Ro RNPs on their chaperone activity. (A) Comparison of the cis-splicing activity of free La and Ro60 with La-hY1 RNA and Ro60-hY1 RNA complexes. Two micromolar protein or pre-bound Y RNA–protein complex were tested. As shown previously free La protein increased cis-splicing (filled blue squares); however, when bound to hY1 RNA the RNA chaperone activity was lost (open blue squares). When Y RNA and La were added to the cis-splicing assay without pre-incubation, La could still stimulate the reaction, however to a lesser extent (filled blue diamonds). Ro60 was inactive and also the association of hY1 RNA did not stimulate the reaction (open red triangle); as expected, hY1 RNA by itself did not show any effect on cis-splicing (open circle). (B) Comparison of trans-splicing activity of free hnRNP I and La protein to pre-bound hnRNP I-hY1 RNA and La-hY1 RNA complexes. The bar graph shows relative splicing rates calculated as described in Figure 2 by the formula (nx − n37)/(n55 − n37), where “nx” is the splicing rate of the respective protein or protein–Y RNA complex, “n55” the splicing rate at 55°C in the absence of protein (left bar), and “n37” the splicing rate at 37°C in the absence of protein (second bar from the left). Only free hnRNP I stimulated trans-splicing, whereas the hY1 RNA–hnRNP I complex was completely inactive. A moderate decrease in splicing was seen when hnRNP I and hY1 RNA were added separately (second bar from the right). No activity was observed for La or La-hY1 RNA complexes.