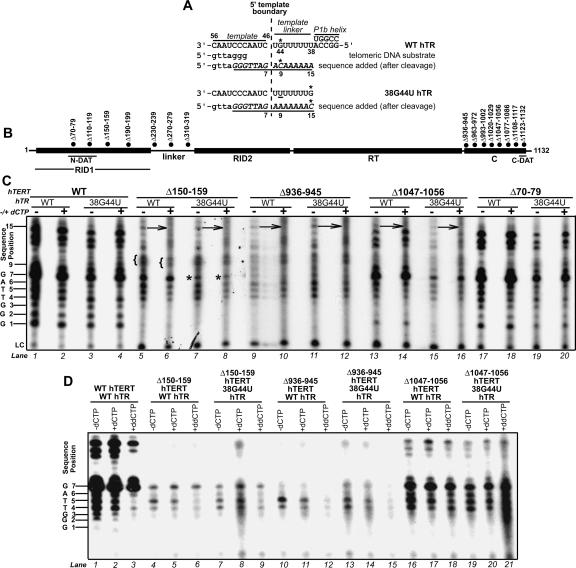
FIGURE 1.
Catalytic phenotypes of hTERT mutants in the presence of the noncognate nucleotide dCTP. (A) Schematic of the hTR template, 5′ template boundary, template linker, and P1b helix. The positions of hTR nt 38, 44, 46, and 56 are indicated. Wild-type (WT) and 38G44U hTRs are illustrated in the upper and lower panels. Alignment of the 3′ end of the DNA substrate with the template is indicated below the schematic for wild-type hTR. The products added to this primer after cleavage (underlined italics) are indicated below the schematics for wild-type and 38G44U hTRs (Huard and Autexier 2004). The positions of products resulting from addition of 7, 9, or 15 nt to the cleaved DNA substrate are indicated. The hTR positions directing dCTP incorporation and the sites of dCTP incorporation in products for wild-type and 38G44U hTRs are marked by asterisks. (B) Schematic illustrating hTERT domains and positions of mutations discussed in this study. (C ) Catalytic phenotypes of selected hTERT mutants reconstituted in RRL with wild-type or 38G44U hTRs, and assayed in the presence (+) or absence (−) of unlabeled dCTP. The dCTP-dependent catalytic phenotypes of these and other hTERT mutants not depicted in this figure are summarized in Table 1. The position numbers of each product are indicated at left. Arrows indicate products at position 15 that exhibit reduced intensity in the presence of dCTP. Opening braces ({) indicate positions at which product mobility is altered in the presence of dCTP. Asterisks indicate positions at which product intensity is reduced when telomerase is assembled with 38G44U hTR in both the presence and absence of dCTP. (LC) Loading control. A lower exposure is shown for the wild-type hTERT samples in lanes 1–4 to facilitate comparison of product pausing patterns. To enhance detection of telomerase activity, the reaction and sample volumes for all C-terminal hTERT mutants and the Δ150–159 variant were scaled up twofold with respect to wild-type hTERT and other mutants in this experiment and all others described in this study. The same amount of loading control was added to each reaction, irrespective of reaction volume. (D) Catalytic phenotypes of selected hTERT mutants reconstituted in RRL with wild-type or 38G44U hTRs and assayed in the absence of dCTP (−dCTP) or in the presence of dCTP (+dCTP) or dideoxy CTP (+ddCTP). Since the overall activity of RID1 and C-terminal mutants was very weak compared to wild-type telomerase, in some experiments such as the one shown here we could not always detect products longer than one telomere repeat. The position numbers of products are indicated at left.