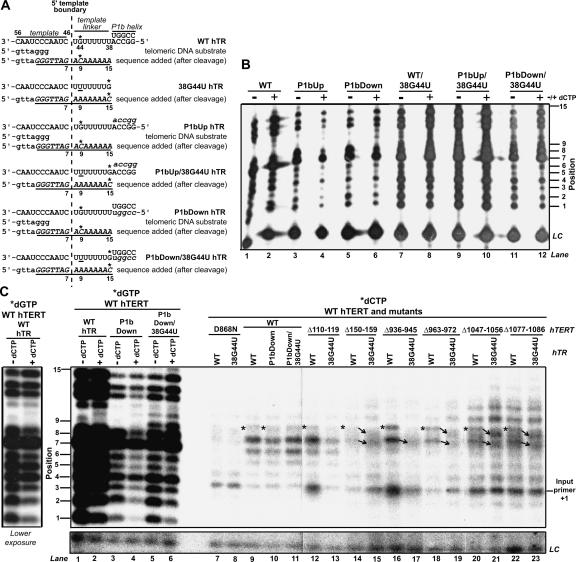
FIGURE 2.
Incorporation of radiolabeled dCTP by hTR P1b and hTERT mutants. (A) Schematic of the positions in wild-type (WT), 38G44U, P1bUp, P1bUp/38G4U, P1bDown, and P1bDown/38G44U hTRs directing incorporation of dCTP (indicated by asterisks). (B) Catalytic phenotypes of hTR P1b mutants reconstituted in RRL with wild-type hTERT. Telomerase assays were performed with radiolabeled dGTP and unlabeled dATP and dTTP in either the presence (+) or absence (−) of unlabeled dCTP. The position numbers of each product are indicated at left. (LC) Loading control. (C) Upper panel: Incorporation of radiolabeled dCTP by hTR mutants assembled with wild-type hTERT and selected hTERT mutants reconstituted with wild-type or 38G44U hTRs. The radiolabeled dCTP incorporation profiles of these and other hTERT mutants not depicted in this figure are summarized in Table 1. The position numbers of each product are indicated at left. Positions at which dCTP should be incorporated following 5′ template boundary read-through in hTERTs assembled with wild-type hTR (position 9) are marked by asterisks. Products whose mobilities were altered when hTERT mutants were reconstituted with wild-type or 38G44U hTRs are indicated by arrows. Telomerase assays performed with a catalytically inactive hTERT RT mutant (D868N) and radiolabeled dCTP indicated that some nonspecific dCTP incorporation occurred at product position 4 (lanes 7,8); nonspecific product patterns generated by D868N telomerase were similar to those observed for hTERT alone (data not shown). This might have been the result of a nonspecific terminal transferase-type activity in RRL, since a product of this size would result if a single labeled nucleotide were added to uncleaved input primer (position indicated by the label “input primer +1”). Control reactions performed with radiolabeled dGTP in the presence (+) or absence (−) of unlabeled dCTP are shown in the first six lanes. A lower exposure of wild-type hTERT samples from lanes 1 and 2 is shown at left to facilitate comparison of product pausing patterns. All reactions were performed in the same experiment and products were electrophoretically separated on the same gel. Lower panel: (LC) loading control. A longer exposure is shown because of weak LC signal in this experiment.