Figure 1.
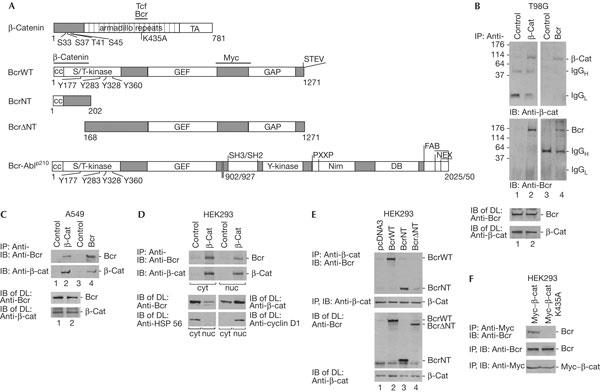
Interaction of Bcr with β-catenin. (A) Schematic diagrams of β-catenin, BcrWT, BcrNT, BcrΔNT and Bcr-Ablp210 constructs used. Bcr is a serine/threonine (S/T) kinase with four tyrosine phosphorylation sites. GEF is a guanine nucleotide exchange factor and GAP is a GTPase-activating protein. Bcr–Abl is a tyrosine (Y) kinase and a fusion protein with various breakpoints of Bcr at 902 or 927. p210 indicates the molecular mass (kDa); SH2 and SH3 are Src-homology domains; PXXP is the proline-rich region; NIM is the nuclear import region; DB is a DNA-binding domain; FAB is an actin binding site; NEX is the nuclear export region. Horizontal bars mark binding regions of the proteins indicated. TA denotes transactivator. (B) T98G is a glioblastoma cell line. Immunoprecipitation (IP) and subsequent immunoblot (IB) were carried out with antibodies as indicated, and with an irrelevant antibody as a control. IgG heavy and light chains (H, L) and molecular mass in kDa are indicated as markers. (C) Same as (B) except using an A549 human lung carcinoma cell line. Protein expression levels were analysed in direct lysates (DL) by IB. (D) (Top) Co-IP of endogenous Bcr with β-catenin from cytoplasmic (cyt) and nuclear (nuc) extracts from human embryonic kidney (HEK)293 cells. IP, IB and DL were carried out as indicated. Cyclin D1 served as a nuclear marker and heatshock protein HSP 56 as a cytoplasmic marker. (E) The plasmids coding for BcrWT, BcrNT and BcrΔNT were transfected into HEK293 cells; pcDNA3 is an empty plasmid control. IPs were carried out with anti-β-catenin, followed by IB with anti-Bcr (N-20 and C-20 together). Protein expression controls are shown below. (F) A Myc-tagged β-catenin wild type and a point mutant of β-catenin (K435A) were expressed ectopically in HEK293 cells. Co-IP and IB were carried out as indicated (top). Protein expression controls are shown below.
