Figure 3.
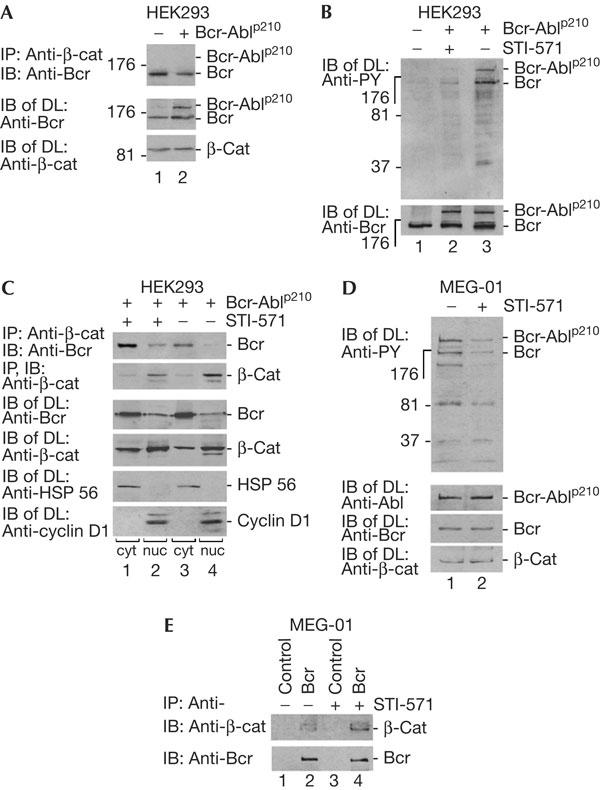
Tyrosine phosphorylation regulates Bcr and β-catenin complex formation. (A) Human embryonic kidney (HEK)293 cells were transfected with Bcr-Ablp210-expressing construct and treated for co-immunoprecipitation (Co-IP), immunoblotting (IB) and direct lysates (DL), as indicated, using Bcr antibodies (N-20), which would also recognize Bcr–Abl. (B) Cells as shown in (A) were treated with STI-571 (2 μM, 1 h). IB was carried out with phosphotyrosinespecific antibodies (PY). For control, Bcr–Abl and Bcr proteins are shown after IB with a Bcr-specific antibody (N-20) in DL. (C) Complex formation regulated by Bcr–Abl kinase. Cells as in (A) were treated with STI-571, fractionated as in Fig 1D and then IP and IB were carried out using Bcr antibodies (C-20). Cell fractionation was controlled by antibodies against HSP 56 and cyclin D1. (D) Human chronic myelogenous leukaemia (CML) cell analysis. The human patient cell line MEG-01 expressing endogenous Bcr and Bcr-Ablp210 was used to detect the phosphorylated forms of proteins by P-Y antibodies in the absence or presence of STI-571 (2 μM, 24 h). Bcr was detected by C-20 antibodies. (E) Co-IP of Bcr (C-20 antibodies) with β-catenin in MEG-01 cells in the presence or absence of STI-571.
