Figure 5.
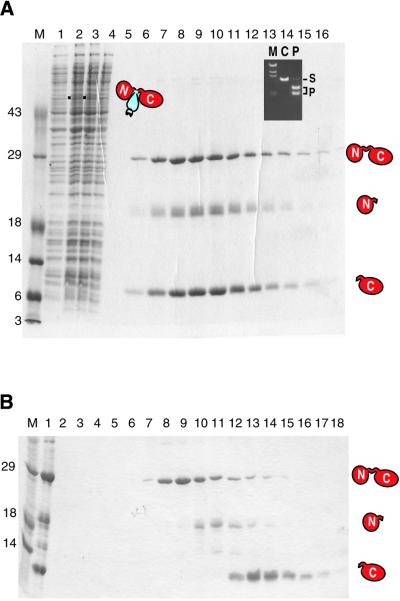
Intein-mediated expression and purification of I-TevI. (A) Affinity column purification by pH shift. The SDS–PAGE shows the efficacy of the purification scheme illustrated in Figure 1. Lane M, protein size markers; lane 1, uninduced sample; lane 2, induced sample, filled square, the fusion precursor Tev::ΔI::CBD of 52.7 kDa; lane 3, clarified cell lysate; lane 4, chitin column flow-through; lanes 5–16, eluted fractions after on-column splicing at pH 7.5 for 26 h at 4°C. The splice product at 28.3 kDa and cleavage products at 18.9 and 9.4 kDa are indicated. The inset shows a DNA cleavage assay with the eluted I-TevI fractions. M, λ DNA HindIII markers; C, control with no enzyme added; S, ScaI-linearized plasmid substrate containing the td homing site; P, cleavage products with purified I-TevI fraction. (B) Gel filtration. The SDS–PAGE gel shows fractions eluted from a Superose 12 gel filtration column. Lane 1, load; lanes 2–18, eluted fractions. Labels as in (A).