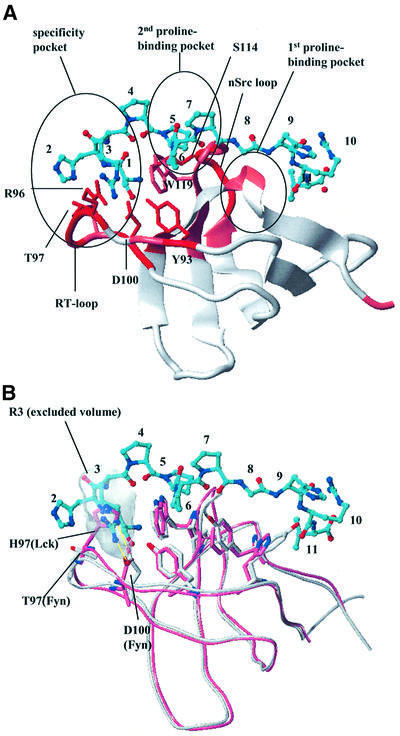
Fig. 4. Fyn SH3 domain binding to the CD2 sequence SHRPPPPGHRV. (A) Model of the complex of the Fyn SH3 domain with the SHRPPPPGHRV ligand of CD2. The ribbon diagram of the SH3 domain is color coded according to the chemical shift perturbations observed in the NMR shift mapping experiments (red, combined chemical shift index >1.0; pink, combined chemical shift index 0.5–1.0). The side chains of residues making direct contacts to the ligand are displayed in red and marked by residue type and number. The ligand is shown as a bonded structure in cyan. Functionally important elements (RT and nsrc loops, specificity pocket and the two proline-binding pockets) of the SH3 domain fold are highlighted. (B) Structural superposition of the Lck (light red) and Fyn SH3 domain (white). The model of the Fyn SH3 domain in complex with the SHRPPPPGHRV peptide from CD2 was overlapped with the Lck SH3 structure (1Lck; Eck et al., 1994). The side chains of residues in the conserved proline-binding pockets are shown to align extremely well for this part of the interaction site. The side chains of RT loop residues that contribute to the observed binding specificity by interacting with (in the case of Fyn) or sterically hindering (in the case of Lck) the insertion of Arg3 of the peptide into the specificity pocket are also displayed. The shaded area defines the exclusion volume of Arg3. As in (A), the ligand is shown as a bonded structure with its carbons colored cyan.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.