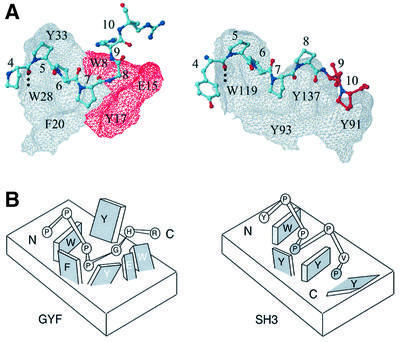
Fig. 5. Comparison of GYF and SH3 domain–ligand interactions. (A) The CD2BP2 GYF domain in complex with the PPPPGHR sequence from CD2 and the Fyn SH3 domain in complex with the peptide YPPPPVP are shown side by side. The surface of the GYF and the SH3 domain is shown as a grid with the residues Trp8, Glu15 and Tyr17 of the GYF domain displayed in red. Conserved residues important for binding are marked by residue type and sequence number. The peptides are shown as a bonded structure with residues 9 and 10 of the Fyn ligand colored red to highlight the extension of the proline helix as compared with the GYF domain ligand. (B) Schematic model showing the two proline-binding sites for the SH3 (right) and the single pocket in the GYF domain (left). Residues of the protein domains are displayed as rectangular structures, with labels for protein residues as either black (conserved domain residues) or white (non-conserved residues). Peptide residues are shown as circles labeled inside by residue type. The two prolines of the PxxP SH3 recognition motif are shaded gray.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.