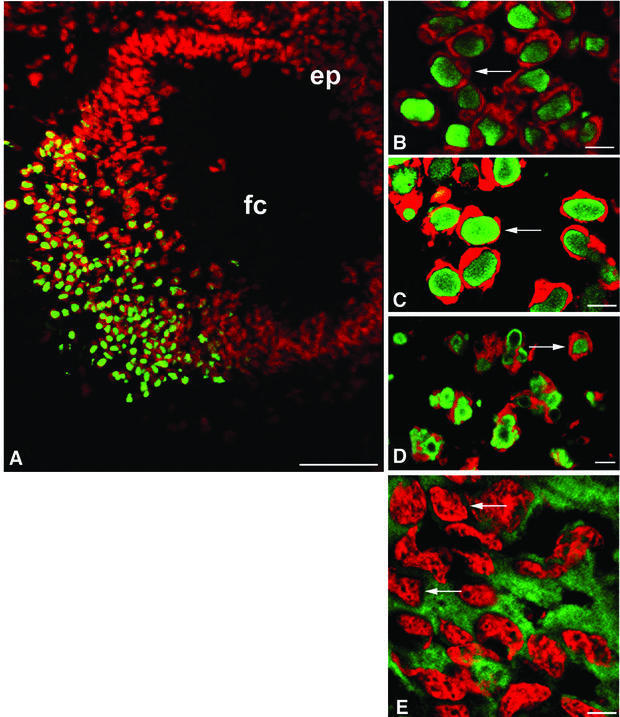
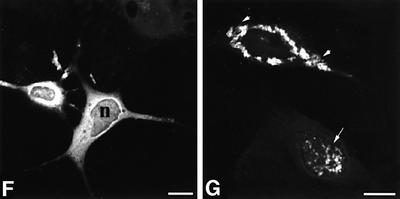
Fig. 2. Nuclear inclusions are common to the three different Cryg cataracts. These murine models of cataract have altered γ-crystallin genes that induce similar structural consequences. Crygbnop is caused by a replacement of 11 bp by 4 bp in the third exon of γB-crystallin at Ser138, generating a unique hexapeptide sequence at the C-terminus of γBnop-crystallin and truncating the 174 amino acid wild-type sequence at residue 144. A C→G transversion in exon 3 accounts for Cryget, which truncates the protein after residue 143 with no changes to the sequence. Crygeelo is characterized by a single base pair deletion in the third exon of Cryge, and this also introduces a unique hendecapeptide sequence before a premature termination at residue 145 for γEelo-crystallin. Lenses from E17.5 mice from Cryget (A and B), Crygeelo (C) and Crygbnop (D) were stained (green channel) with polyclonal specific antibodies to all γ-crystallins (A and B), γEelo-crystallin (C) and γBnop-crystallin (D), and also counterstained with propidium iodide (red channel) to highlight the nuclei. Ep, lens epithelium; fc, lens fibre cells. Scale bars = 50 µm in (A) and 5 µm in (B–D). (E) Staining of E17.5 lens from a wild-type mouse using polyclonal antibodies that detect all six mouse γ-crystallins (green channel), counterstained with propidium iodide (red channel). Scale bar = 5 µm. Ptk2 cells were transiently transfected with wild-type Crygb (F) and Crygbnop (G) both fused to GFP tags to examine the role of the lens environment in nuclear-specific location of the inclusions. Wild-type γB-crystallin is found in both the nuclei (n) and cytoplasm of cells, but does not form protein inclusions. γBnop-crystallin forms both nuclear (arrows) and cytoplasmic inclusions (arrowheads) in transfected cells, the two extremes captured in this image (G). Transfected cells containing both cytoplasmic and nuclear inclusions were a more usual observation. Scale bars = 10 µm.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.