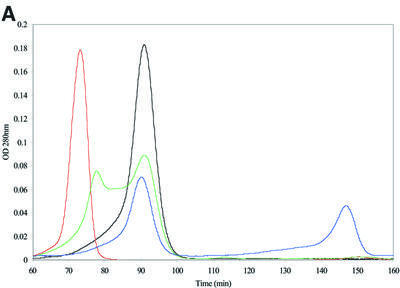
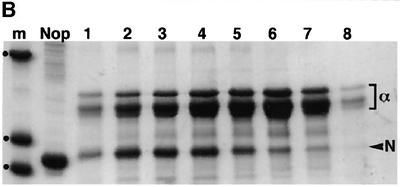
Fig. 3. α-crystallin prevents Crygbnop aggregation in vitro. (A) Recombinant γBnop-crystallin (red chromatogram) elutes in the void volume of a BioSec size exclusion column indicating aggregation to a size significantly bigger than the molecular weight of the monomeric protein (∼20 kDa). α-crystallin (black chromatogram), which oligomerizes in vivo to form particles of 800 kDa, elutes after the γBnop- crystallin peak. Wild-type mouse γ-crystallins and α-crystallins elute independently when separated on the same column (blue chromatogram): the γ-crystallins elute after 148 min, equivalent to their mol. wt of 20 kDa, whereas the α-crystallins elute at 91 min. When purified γBnop-crystallin was mixed in equimolar amounts with α-crystallin, two peaks were observed (green chromatogram). One peak has an equivalent elution time to α-crystallin, whilst the other peak is slightly smaller and shifted from the position seen for γBnop-crystallin alone (major peak in the red chromatogram). OD values have been adjusted relative to those obtained for α-crystallin to allow overlay of the different chromatograms. (B) The protein content of the two peaks from the green chromatogram was determined by SDS–PAGE, and each peak contains both α-crystallin (α) and γBnop-crystallin (N). This shows that γBnop-crystallin and α-crystallins co-elute, indicating that a protein complex has formed between these proteins. Proteins in fractions after 70, 72, 78, 82, 86, 90, 94 and 98 min are shown in tracks 1–8, respectively. Track M contains marker proteins of 30, 17.2 and 12.3 kDa, indicated by dots. The purity of the starting material γBnop-crystallin is shown (track Nop).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.