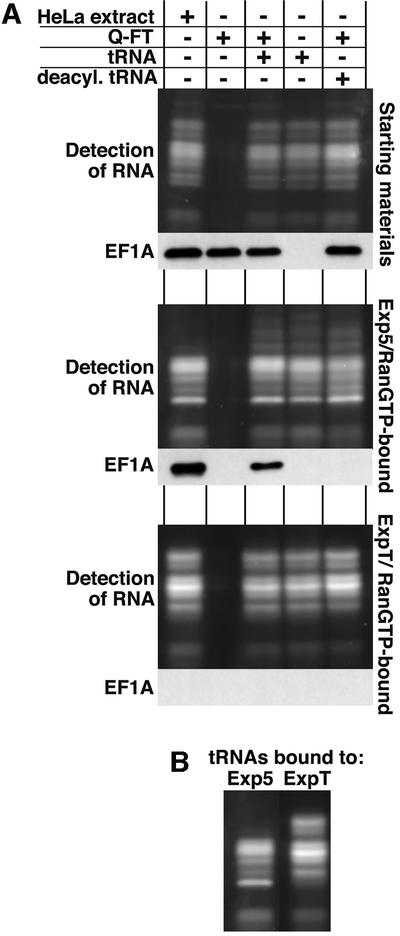
Fig. 5. The interaction between Exp5 and eEF1A is mediated by tRNA. (A) A cytosolic HeLa extract was depleted of nuclear transport receptors and fractionated further to separate eEF1A from tRNA: the flowthrough from a Q-Sepharose column contained eEF1A, but lacked tRNA, while the phenol/chloroform extract contained tRNA, but no protein. Where indicated, tRNA was used after deacylation. These fractions were tested singly or in combination for binding to Exp5/RanGTP or ExpT/RanGTP as in Figure 1. The figure shows starting materials (upper parts), Exp5-bound materials (middle parts) and ExpT-bound materials (lower parts). Detection of eEF1A was by western blotting. RNA was detected after Sybr-green staining of the 12% polyacrylamide gel. The load of the bound fractions corresponds to 10 times the starting materials. Note that eEF1A binding to Exp5 was strictly RNA dependent, but tRNA binding to Exp5 occurred independently of eEF1A. Deacylation of the tRNA (which prevents the tRNA–EF1A interaction) only abolished recruitment of eEF1A into Exp5 export complexes, but still allowed efficient tRNA binding to either Exp5 or ExpT. (B) A comparison of RNA patterns when bound to Exp5 and Exp-t.
