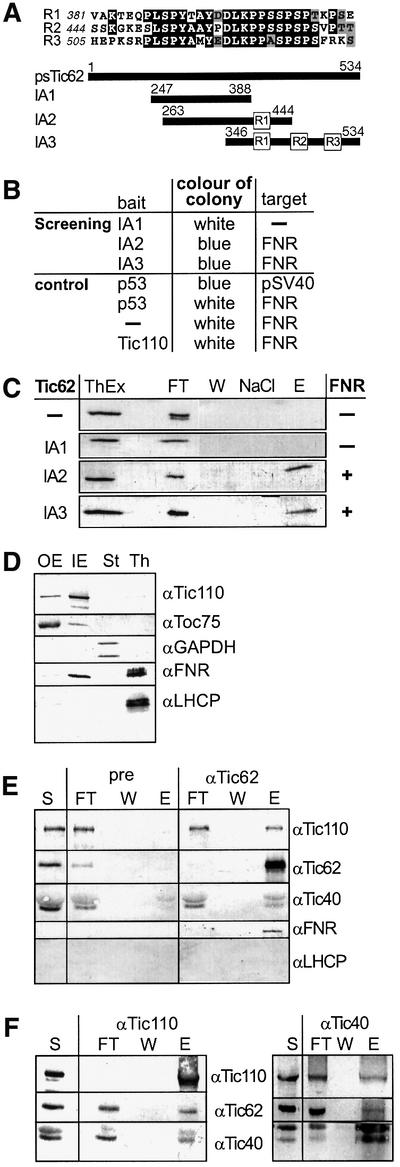
Fig. 5. psTic62 interacts with FNR. (A) Comparison of the repetitive modules at the C-terminus of psTic62. The numbers indicate the position of the amino acids. A scheme of the polypeptides used to investigate the interaction of the C-terminus of psTic62 with partner proteins is presented. (B) Overview of the results of the yeast two-hybrid screening. The bait and the target proteins obtained after screening or used for control are shown. (C) Interaction of polypeptides containing the repetitive module with FNR. Proteins extracted from thylakoids (ThEx) were mixed with recombinant C-terminal stretches of psTic62 (see A). After metal affinity chromatography, FNR co-purifies with IA2 and IA3. As a control, only the column material (–) was incubated with the thylakoid extract. The samples were subjected to SDS–PAGE and transferred to nitrocellulose. An immunodecoration with antiserum against FNR is shown. Flow through, FT; washing step, W; washing with NaCl, NaCl; eluted proteins, E. (D) Localization of FNR in chloroplasts. Intact chloroplasts were fractionated, and outer (OE) and inner envelope (IE) membranes, thylakoids (Th) and stroma (St) (10 µg of protein) were subjected to SDS–PAGE. Proteins were transferred to nitrocellulose and immunodecorated with antisera against Toc75 (outer envelope), Tic110 (inner envelope), GAPDH (stroma), FNR and LHCP (thylakoids). (E) Inner envelope vesicles (400 µg of protein) were solubilized with 3% decyl maltoside. After centrifugation, the supernatant was incubated with Tic62 antibodies coupled to AF-Tresyl 650M. Aliquots of the supernatant (S), the flow through (FT) of the Tic62 column, the last washing step (W) and the eluted (E) protein (1/5) were subjected to SDS–PAGE and transferred to nitrocellulose. Antisera raised against Tic110, Tic62, Tic40 and FNR were used to analyse the co-immunoprecipitated proteins. (F) Co-immunoprecipitation of the Tic complex with antibodies against different Tic components. Inner envelope vesicles were solubilized with 3% decyl maltoside. After centrifugation, the supernatant was incubated with Tic110 antibodies coupled to AF-Tresyl 650M (left panel) or Tic40 antibodies coupled to protein A–Sepharose (right panel). Aliquots of the supernatant (S), the flow through (FT), the last washing step (W) and the eluted protein (E) were subjected to SDS–PAGE and transferred to nitrocellulose. Antisera raised against Tic110, Tic62 and Tic40 were used to analyse the co-immunoprecipitated proteins.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.