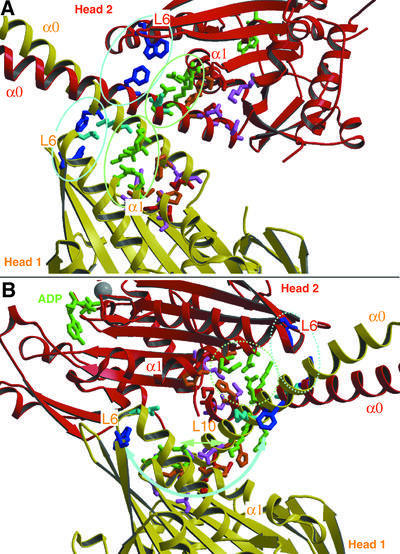
Fig. 4. The X-ray structure of dimeric ncd motor domains revealed a number of interactions between the motor cores (α1, loops 6, 10 and 13) and their corresponding neck helices (α0). (A) In our model, based on the docking shown in Figure 3, these interactions remain conserved for both heads in ncd dimers bound to MTs in the absence of nucleotide. (B) However, in the presence of AMP-PNP, these contacts may be only conserved for head 2, but not for head 1. Potential contacts now appear between loops 6 and 10 in head 1, and loop 2, β1a/b and the beginning of α1 in head 2. This indicates that ATP uptake triggers a conformational change within the motor core domain, which directly influences the neck–core interaction, and which may therefore constitute the underlying mechanism for ncd function.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.