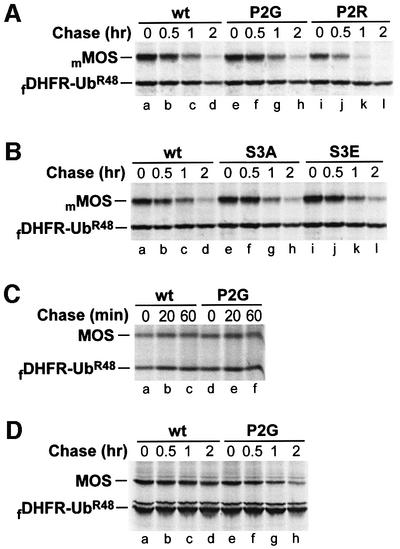
Fig. 7. Degradation of either Xenopus or mouse MOS in mouse NIH 3T3 cells, Xenopus XTC cells and rabbit reticulocyte lysate does not depend on the N-terminal Pro residue of MOS. Positions of fDHFR-UbR48 and either Xenopus MOS (MOS) or mouse MOS (mMOS) are indicated on the left. (A) Pulse–chase in mouse NIH 3T3 cells. Lanes a–d, Pro-Ser-mMOS; lanes e–h, Gly-Ser-mMOSP2G; lanes i–l, Arg-Ser-mMOSP2R. (B) Mutations at Ser-2 (encoded Ser-3) have a negligible effect on degradation of mouse MOS in NIH 3T3 cells. Lanes a–d, Pro-Ser-mMOS; lanes e–h, Pro-Ala-mMOSS3A; lanes i–l, Pro-Glu-mMOSS3E. (C) Xenopus MOS is long-lived in Xenopus XTC cells. Lanes a–c, Pro-Ser-MOS; lanes d–f, Gly-Ser-MOSP2G. (D) N-terminal Pro of Xenopus MOS does not metabolically destabilize MOS in rabbit reticulocyte lysate. Lanes a–d, Pro-Ser-MOS; lanes e–h, Gly-Ser-MOSP2G.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.