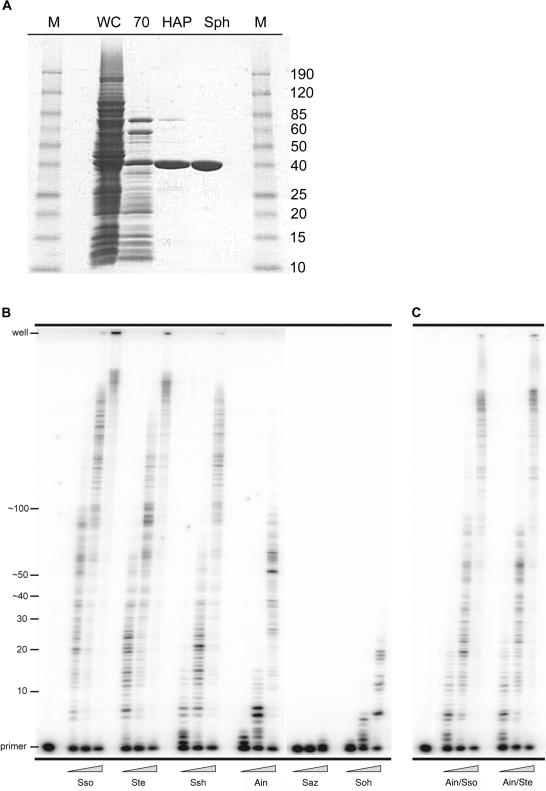
Figure 2.
Purification and processivity of Dpo4-like proteins. (A) Scheme for the purification of Dpo4-like proteins. Whole-cell extracts (WC) were clarified and then heat treated at 70°C for 10 min. Denatured E.coli proteins were removed from the extracts by centrifugation (70). Subsequently, the soluble extracts were passed over a Hydroxyapatite Bio-Gel HTP Gel column (HAP) and then a SP Sepharose HP column (Sph). The Saz and Soh Dpo4-like proteins were purified over one additional column, a HiLoad 26/60 Superdex 75 column (data not shown). M designates the Benchmark prestained protein size marker (Invitrogen). (B) M13mp18 primer extension reactions using the previously identified S.solfataricus Dpo4 (Sso) and the five newly identified polymerases; S.tengchongensis (Ste), S.shibatae (Ssh), A.infernus (Ain), S.azoricus (Saz) and S.ohwakuensis (Soh). Primer extension of primer M13HTP (5′-CCT TAG AAT CCT TGA AAA CAT AGC GA-3′) annealed to M13mp18 from base pairs 4101 to 4126 was performed at 60°C for 5 min utilizing 10 nM of the M13HTP/M13mp18 primer-template and increasing concentrations of the Dpo4-like enzymes; from left to right 0.2, 2.0 and 20 nM. Replication products were separated on 12%/8 M urea polyacrylamide gels and visualized by PhosphorImager analysis. Primer location and number of nucleotides added are indicated to the left of the gel. (C) M13mp18 primer extension reactions using the ‘little finger’ domain chimeric polymerases; A.infernus/S.solfataricus (Ain/Sso) and A.infernus/S.tengchongensis (Ain/Ste).