Abstract
Studies to measure human gastric crypt or gland cell proliferation may have a number of practical clinical applications in relation to both benign and malignant gastric conditions. Bromodeoxyuridine (BrdUrd) labels human gastric mucosal cells in the S phase. Computer aided data analysis of labelled mucosa allows static proliferative indices to be estimated, including the crypt labelling index (LI), the peak labelling position, the distribution of labelled cells and indirectly the crypt growth fraction. Multiparameter flow cytometric analysis of labelled nuclei allows the S phase duration (Ts) of mucosal cells to be estimated. Specimens of histologically normal gastric body (GB, n = 16) and antral mucosa (GA, n = 10) were obtained from 25 patients with gastric carcinomas who received a bolus dose of 250 mg BrdUrd between 3.0 and 15.7 hours before surgery. Tissue sections were stained by an immunohistochemical method and subjected to detailed counting of up to 50 longitudinal crypts per specimen. The total crypt labelling index was calculated by a grid counting method. A significant difference existed between the proliferative compartments of gastric antral and body mucosa measured by a number of criteria. The median lengths of the crypts were 137 cells (GB) and 188 cells (GA). The median peak labelling positions were cell 26 (GB) and cell 61 (GA) from the crypt orifice. The mean crypt labelling indices were 2.8% (GB) and 4.8% (GA). The mean Ts of GA cells was 7.7 hours and of GB cells was 10.8 hours.
Full text
PDF
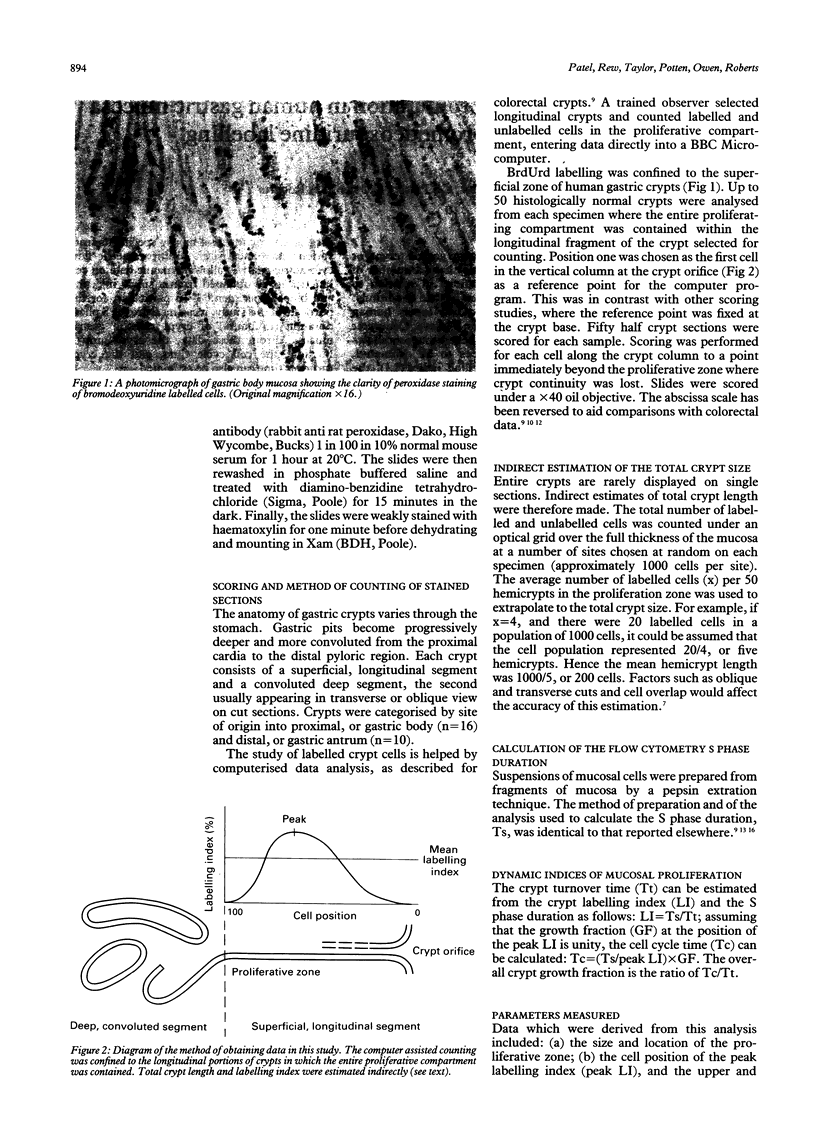
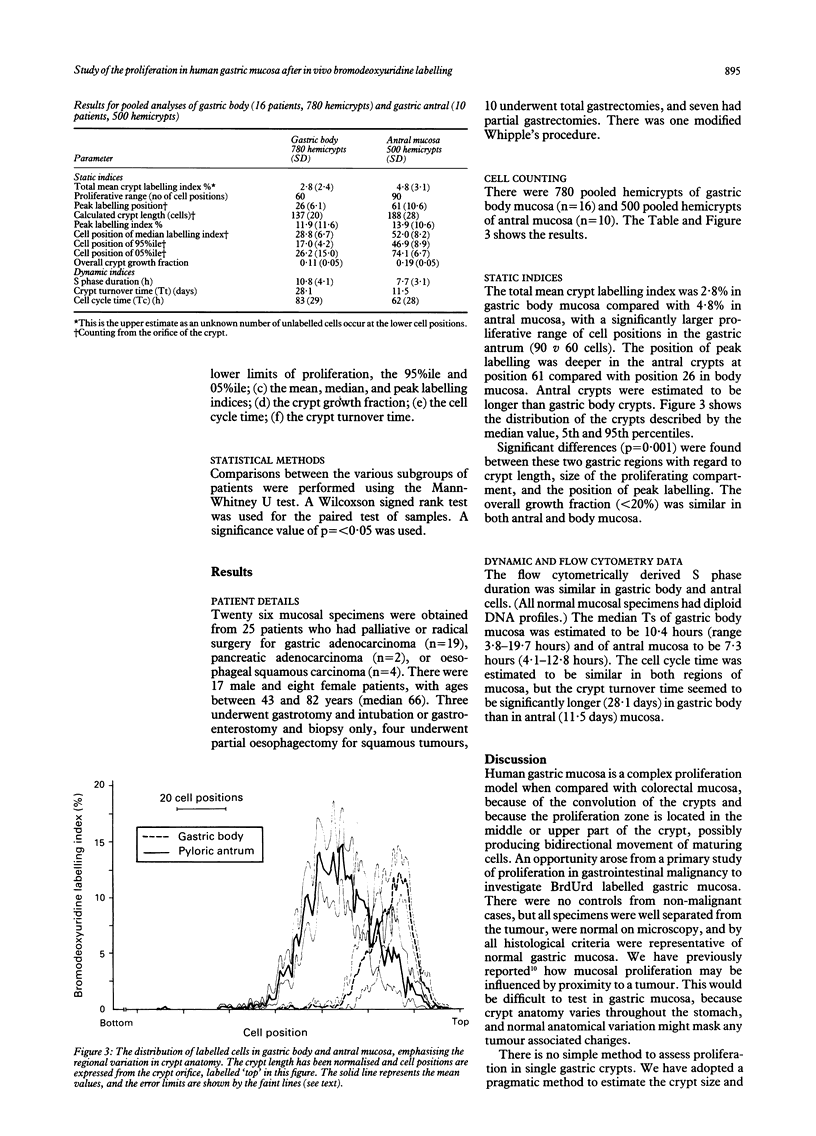

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg A. C., McNally N. J., Shrieve D. C., Kärcher H. A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry. 1985 Nov;6(6):620–626. doi: 10.1002/cyto.990060618. [DOI] [PubMed] [Google Scholar]
- Chwalinski S., Potten C. S., Evans G. Double labelling with bromodeoxyuridine and [3H]-thymidine of proliferative cells in small intestinal epithelium in steady state and after irradiation. Cell Tissue Kinet. 1988 Sep;21(5):317–329. doi: 10.1111/j.1365-2184.1988.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Kamata T., Yonemura Y., Sugiyama K., Ooyama S., Kosaka T., Yamaguchi A., Miwa K., Miyazaki I. Proliferative activity of early gastric cancer measured by in vitro and in vivo bromodeoxyuridine labeling. Cancer. 1989 Oct 15;64(8):1665–1668. doi: 10.1002/1097-0142(19891015)64:8<1665::aid-cncr2820640818>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kovacs L., Potten C. S. An estimation of proliferative population size in stomach, jejunum and colon of DBA-2 mice. Cell Tissue Kinet. 1973 Mar;6(2):125–134. doi: 10.1111/j.1365-2184.1973.tb01601.x. [DOI] [PubMed] [Google Scholar]
- LIPKIN M., SHERLOCK P., BELL B. CELL PROLIFERATION KINETICS IN THE GASTROINTESTINAL TRACT OF MAN. II. CELL RENEWAL IN STOMACH, ILEUM, COLON, AND RECTUM. Gastroenterology. 1963 Dec;45:721–729. [PubMed] [Google Scholar]
- Lipkin M., Higgins P. Biological markers of cell proliferation and differentiation in human gastrointestinal diseases. Adv Cancer Res. 1988;50:1–24. doi: 10.1016/s0065-230x(08)60433-9. [DOI] [PubMed] [Google Scholar]
- Morris S. M. The genetic toxicology of 5-bromodeoxyuridine in mammalian cells. Mutat Res. 1991 Sep;258(2):161–188. doi: 10.1016/0165-1110(91)90007-i. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Kellett M., Rew D. A., Roberts S. A. Proliferation in human gastrointestinal epithelium using bromodeoxyuridine in vivo: data for different sites, proximity to a tumour, and polyposis coli. Gut. 1992 Apr;33(4):524–529. doi: 10.1136/gut.33.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Kellett M., Roberts S. A., Rew D. A., Wilson G. D. Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut. 1992 Jan;33(1):71–78. doi: 10.1136/gut.33.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems G., Galand P., Chretien J. Autoradiographic studies on cell population kinetics in dog gastric and rectal mucosa. A comparison between in vitro and in vivo methods. Lab Invest. 1970 Dec;23(6):635–639. [PubMed] [Google Scholar]
- Willems G., Galand P., Vansteenkiste Y., Zeitoun P. Cell population kinetics of zymogen and parietal cells in the stomach of mice. Z Zellforsch Mikrosk Anat. 1972;134(4):505–518. doi: 10.1007/BF00307670. [DOI] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dische S., Saunders M. I., Des Rochers C., Lewis A. A., Bennett M. H. Measurement of cell kinetics in human tumours in vivo using bromodeoxyuridine incorporation and flow cytometry. Br J Cancer. 1988 Oct;58(4):423–431. doi: 10.1038/bjc.1988.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dunphy E., Kärcher H., Pfragner R. The labelling index of human and mouse tumours assessed by bromodeoxyuridine staining in vitro and in vivo and flow cytometry. Cytometry. 1985 Nov;6(6):641–647. doi: 10.1002/cyto.990060621. [DOI] [PubMed] [Google Scholar]
- Winawer S. J., Lipkin M. Cell proliferation kinetics in the gastrointestinal tract of man. IV. Cell renewal in the intestinalized gastric mucosa. J Natl Cancer Inst. 1969 Jan;42(1):9–17. [PubMed] [Google Scholar]



