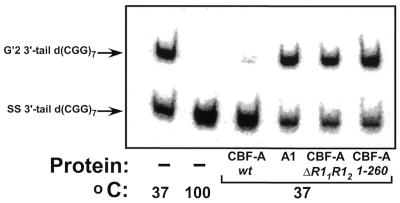
Figure 4.
Destabilization of G′2 3′-tail d(CGG)7. Wild-type CBF-A or hnRNP A1 proteins, at 33 pmol each or 165 pmol each of R11R12 or 1–260 CBF-A mutant proteins were incubated at 37°C for 15 min with 0.16 pmol of 5′-32P G′2 3′-tail d(CGG)7 under standard tetraplex DNA destabilization assay conditions. Control samples that did not contain protein were similarly incubated at 37 or 100°C to reveal the initial amount of G′2 3′-tail d(CGG)7 and the position of displaced single-stranded DNA, respectively. The tetraplex unwinding reaction was terminated by the addition of 1% SDS to a final concentration of 0.3%, and tetraplex and single-stranded 3′-tail d(CGG)7 were resolved by electrophoresis through a 10% polyacrylamide gel, 0.5× TBE buffer, 10 mM KCl. A phosphorimage of the electropherogram is shown.