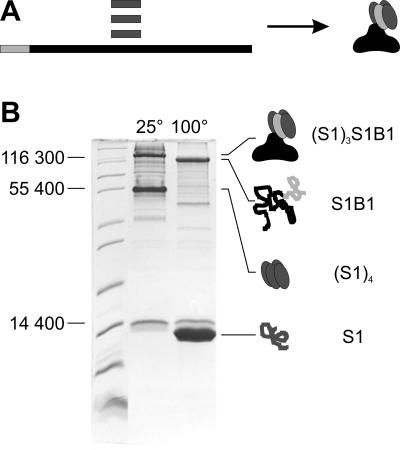
Fig 1.
Refolding of heterotetrameric fusion protein (S1)3S1B1 (A) and confirmation by SDS/PAGE analysis (B). (A) Core streptavidin (S1, dark gray) and fusion protein S1B1 (streptavidin moiety S1, light gray; SbsB moiety B1, black) were produced in E. coli and isolated independently, mixed, and refolded to heterotetramers (S1)3S1B1. (B) For SDS/PAGE analysis, samples were recovered from a refolding batch, and SDS was added to a concentration of 5%. Without boiling of the sample (lane 25°C), streptavidin–SbsB heterotetramers (S1)3S1B1 (144,973 Da) and core streptavidin homotetramers (S1)4 (49,840 Da) migrated at apparent molecular masses of 125,000 and 55,000 Da, respectively, on an SDS/15% PAGE gel. After boiling (lane 100°C), the tetramers of both the fusion protein and core streptavidin were denatured to monomers (S1B1, 107,593 Da; S1, 12,460 Da).