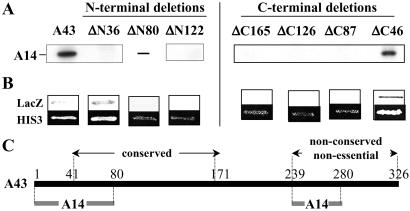
Fig 3.
Interaction domains of subunit A43 with subunit A14. (A) Interaction of A14 subunit with hemagglutinin-tagged A43 polypeptides progressively truncated at the N terminus (A43-ΔN) or at the C terminus (A43-ΔC) (the number of deleted residues is indicated). Each truncated form was synthesized in vitro in the presence of [35S]methionine (no protein was obtained for the ΔN80 construction), and their interaction with 35S-A14 was monitored by immunoprecipitation with anti-hemagglutinin antibodies. Immunoprecipitated proteins were separated by SDS/PAGE and visualized by autoradiography. (B) The ORFs encoding the truncated versions of subunit A43 were cloned in fusion with Gal4DBD in pGBT9 vector. Their ability to interact with A14 fused to Gal4AD was monitored in Y190 strain. Activation of the LacZ and HIS3 reporter genes was monitored by staining in the presence of X-gal and by analysis of growth in the presence of 50 mM 3-aminotriazol, respectively. (C) Schematic representation of the interaction domains of A43 with A14. The conserved domain of A43 (residues 42–168), present in putative orthologues of this subunit in Schizosaccharomyces pombe, Candida albicans, and higher eukaryotes (see ref. 22), is indicated. The 87 C-terminal residues of A43 are not conserved through evolution (data not shown). These residues are not essential for cell viability (see Fig. 2) but contain an interaction domain with A14.