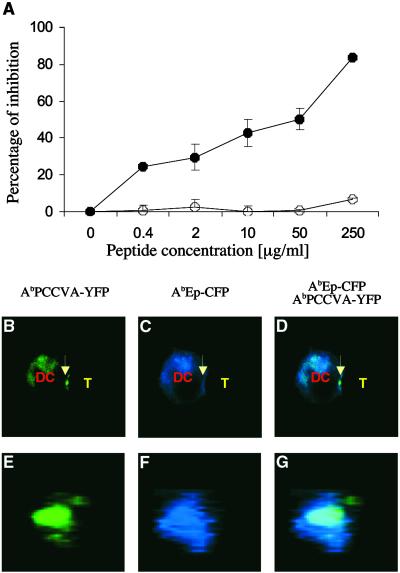
Fig 5.
Antagonist peptide, which inhibits the proliferation of naive TCRTg CD4+ T cells to agonist peptide, also inhibits expulsion of null MHC class II/peptide complexes from the center of the T cell–DC contact site. AbIi− DCs expressing agonist AbPCCVA-YFP and null AbEp-CFP complexes were preincubated with the indicated concentration of antagonist PCC50E (•) or null PCC52Q (○) peptides (A) or with 50 μM antagonist PCC50E peptide (B–G) and cultured with naive TCRTg CD4+ T cells. (A) Inhibition of [3H]thymidine incorporation ± SD into proliferating T cells (representative of three independent experiments) is shown. (B–D) A single 2D optical section in the center of the T cell–DC conjugate. (E–G) A 3D view of the T cell–DC contact. (B and E) AbPCCVA-YFP only (green). (C and F) AbEp-CFP only (blue). (D and G) an AbPCCVA-YFP and AbEp-CFP overlay. Agonist AbPCCVA complexes were clustered in 61% of recorded contacts between DCs and T cells. Only 11% of the clusters were characterized by the exclusion of neutral AbEp-CFP complexes (Table 1). Expulsion of a particular complex was defined by at least a 50% decrease in the intensity of fluorescence in comparison with the intensity in the proximity of the place of contact. T cell–DC contacts (103 in total) were analyzed in three independent experiments.