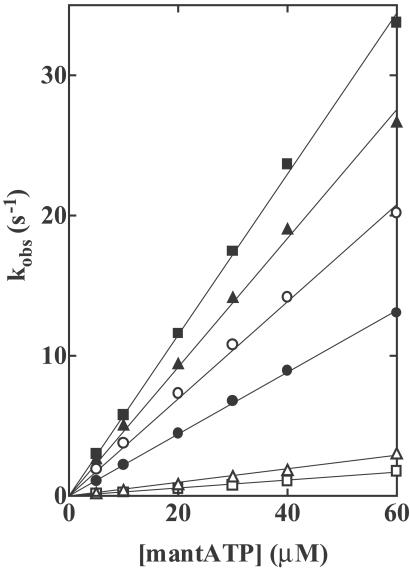
Fig 1.
Rates of binding of mantATP to wild-type and mutant HMMs. Various concentrations of mantATP were mixed with 0.56 μM HMMs in a rapid mixing stopped flow fluorometer, and the increases in the energy transfer from Trp residues to bound mantATP were recorded. Observed rate constants kobs were plotted as a function of the mantATP concentration. The slope of the plots of kobs vs. [mantATP] are the second-order rate constants of mantATP binding (K1k+2) and are listed in Table 1. HMMs: Wild-type (○), R247A (▵), R247E (□), E470A (▴), E470R (▪), and R247E/E470R (•). Conditions: 0.45 M KCl, 3 mM MgCl2, 1 mM EDTA, 20 mM Tris⋅HCl (pH 7.5) at 20°C.