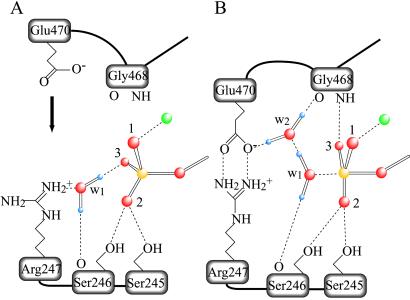
Fig 5.
Schematic representation of the γ-phosphate pocket of the prehydrolytic state (A) and of the transition state for hydrolysis (B). In the former, the γ-phosphate of ATP is in a tetrahedral arrangement with its four oxygen atoms, whereas, in the latter, five oxygen atoms bound to the γ-phosphate adopt a trigonal bipyramidal arrangement. Two water molecules involved in hydrogen bonding are denoted as w1 and w2. We assume that w1 in A is HOH1181 in the Fisher et al. structure (ref. 20; Protein Data Bank ID code ), and that w1 and w2 in B take the place of the terminal oxygen of the vanadate moiety and that of HOH697, respectively, in the Smith and Rayment structure (ref. 5; Protein Data Bank ID code ). Only those stereochemical interactions that participate in positioning w1, w2, and three oxygen atoms (labeled 1, 2, and 3) of the γ-phosphate are depicted. Covalent bonds are shown as solid lines and hydrogen bonds and ionic interactions in dashed lines. Phosphorus, oxygen, hydrogen, and magnesium are depicted in yellow, red, violet, and green, respectively.