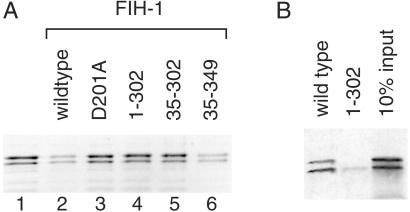
Fig 5.
The C terminus of FIH-1 is required for activity. (A) In vitro hydroxylation of HIF-2α 774–874 by wild-type FIH-1 inhibits interaction with p300. 35S-labeled HIF-2α 774–874 was incubated in the absence (lane 1) or presence of various recombinant MBP-FIH-1 enzymes (lanes 2–6) followed by incubation with immobilized GST-p300 CH1. 35S-labeled HIF-2α 774–874 bound to the GST-p300 CH1 domain was visualized by phosphorimaging after SDS/PAGE. Mutations that interfere with Fe(II) binding (D201A) or delete residues 303–349 compromise FIH-1 ability to block p300 association with the HIF-2α CTAD. (B) Deletion of FIH-1 residues 303–349 prevents interaction with the CTAD. 35S-labeled HIF-2α 774–874 bound to immobilized wild-type or truncated (1–302) MBP-FIH-1 was visualized after SDS/PAGE. The right lane indicates 10% of the input 35S-labeled protein in the pull-down experiments.