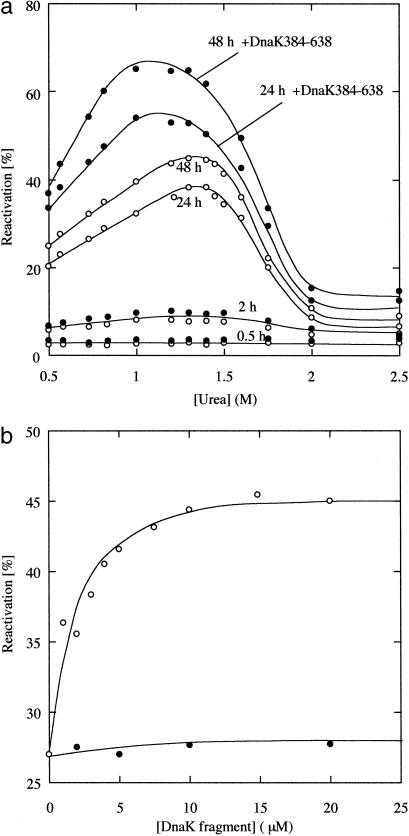
Fig 3.
The reactivation yields of β-galactosidase. β-Galactosidase was denatured with 8 M urea and diluted into refolding buffer (0.1 M sodium phosphate/1 mM MgCl2/5 mM EDTA). Refolding was achieved by incubation for 30 min at 10°C followed by a shift to 20°C to reconstitute the active tetramer. The final concentration of β-galactosidase was 3.4 μM (monomer concentration). (a) The reactivation yields at various concentrations of urea in the absence (○) and presence (•) of 10 μM DnaK384-638. At these urea concentrations, DnaK384-638 was confirmed to be in the native conformation by CD. (b) Concentration dependence of the yield of the reactivity of β-galactosidase at 0.73 M urea in the presence of DnaK384-638 (○) and DnaK386-561 (•). The extent of the reactivation was measured 24 h after refolding.