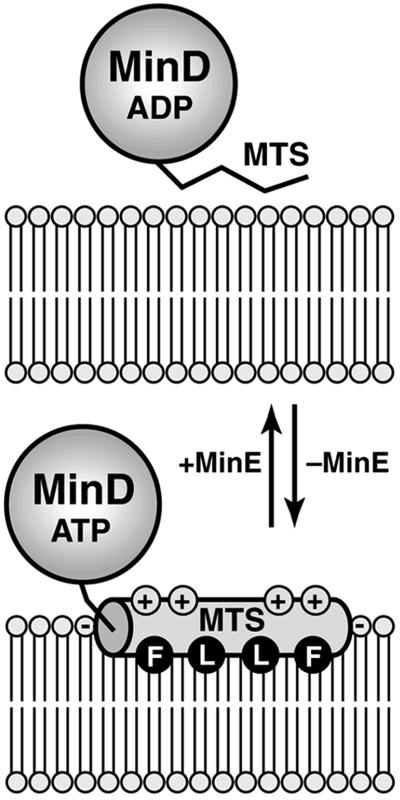
Fig 5.
Model of the MinD membrane attachment–detachment cycle. In the absence of MinE (Lower), the C-terminal MTS of MinD forms an amphipathic helix (shaded cylinder) that interacts with the lipid bilayer. The helix most likely orients parallel to the membrane surface. Partial insertion of the helix into the cytoplasmic monolayer would allow residues such as Phe (F) and Leu (L) on the hydrophobic face of the amphipathic helix to interact with lipid acyl chains, whereas residues on the opposing polar face of the helix could interact with lipid headgroups. The numerous cationic residues (indicated by +) on the polar face of the MinD MTS probably make specific contacts with the headgroups of anionic phospholipids (indicated by −). We presume that MinE causes detachment of the MTS, thus releasing MinD from the membrane (Upper). It is unclear at present whether this release involves a direct interaction between MinE and the MTS, a conformational change in MinD provoked by MinE-induced ATP hydrolysis, or some other mechanism.