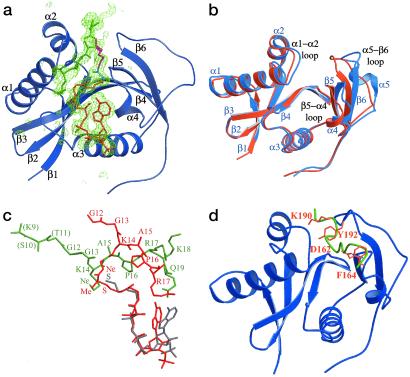
Fig 2.
Structure of the tGCN5/H3-(Me)CoA-20 inhibitor complex. (a) The protein is colored blue with secondary structural elements labeled. The inhibitor is color-coded as described in Fig. 1. A σA-weighted Fo − Fc omit map, omitting the entire inhibitor is shown. The map is contoured to 2.5 σ. (b) Superposition of the ternary tGCN5/CoA/histone H3 peptide complex protein (blue) and the tGCN5/H3-(Me)CoA-20 inhibitor complex protein (orange). (c) Superposition of the ternary (CoA in gray, H3 in green) and inhibitor (red) complex substrates. The respective proteins of the two complexes were used to align the superposition. (d) Superposition of the histone H3 peptide extracted from the ternary tGCN5/CoA/histone H3 complex (green) onto the protein component of the tGCN5/H3-(Me)-CoA-20 inhibitor complex (blue). The respective proteins of the two complexes were used for the superposition. Residues Asp-162 and Phe-164 of the β5−α4 loop and residues Lys-190 and Tyr-192 of the β6−α5 loops are in orange.