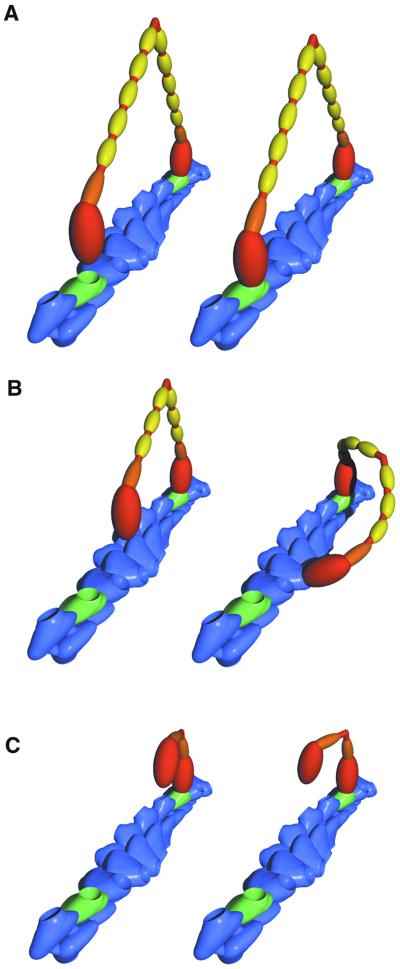
Fig 5.
Model of myosin V stepping. A short segment of an actin filament is shown in blue, with the 36-nm pseudorepeat highlighted by green subunits. Each actin subunit has a single myosin binding site depicted as a pit. Each myosin is colored to show the heavy chain including the catalytic domain (red), the essential light chain (orange), and the calmodulin light chains (yellow). (A) The rear head of a 6IQ-HMM in a poststroke state, tightly bound to an actin subunit (Left). The near head is not yet bound, but in its diffusional search it can easily bind to the green monomer (Right). (B) The smaller stroke of the bound head of the 4IQ-HMM is shown (Left). The unbound head is not positioned so that a binding site is immediately available. The geometry of the actin filament dictates that some portion of the myosin molecule must distort to bind both heads at once. It is possible that the lever arm elastically bends to achieve this conformation (Right). (C) The 1IQ-HMM is shown bound by one head to an actin filament. The second head can bind only if considerable distortion exists in the myosin. These extreme conformations are likely to be rare, accounting for the fact that the second head binds to actin many orders of magnitude slower than the first head.