Abstract
This study is aimed at identifying the Na pump isoform composition of human erythroid precursor cells and mature human erythrocytes. We used purified and synchronously growing human erythroid progenitor cells cultured for 7–14 days. RNA was extracted from the progenitor cells on different days and analyzed by RT-PCR. The results showed that only the α1, α3, β2, and β3 subunit isoforms and the γ modulator were present. Northern analysis of the erythroid progenitor cells again showed that β2 but not β1 or α2 isoforms were present. The erythroid cells display a unique β subunit expression profile (called β-profiling) in that they contain the message for the β2 isoform but not β1, whereas leukocytes and platelets are known to have the message for the β1 but not for the β2 isoform. This finding is taken to indicate that our preparations are essentially purely erythroid and free from white cell contamination. Western analysis of these cultured progenitor cells confirmed the presence of α1, α3, (no α2), β2, β3, and γ together now with clear evidence that β1 protein was also present at all stages. Western analysis of the Na pump from mature human erythrocyte ghosts, purified by ouabain column chromatography, has also shown that α1, α3, β1, β2, β3, and γ are present. Thus, the Na pump isoform composition of human erythroid precursor cells and mature erythrocytes contains the α1 and α3 isoforms of the α subunit, the β1, β2, and β3 isoforms of the β subunit, and the γ modulator.
In a previous article (1), we analyzed human reticulocyte RNA to determine which isoforms of the various Na pump subunits were present. By using the methods of RT-PCR, we found α1, α3, β2, β3, and γ, but not α2 or β1. The present study extends this work by identifying the various subunit isoforms, by Northern and Western blot analyses, in erythroid progenitor cells at different stages of differentiation, as well as by determining which Na pump proteins are present in mature erythrocytes (i.e., mature red blood cells) taken from the peripheral circulation. A primary result is that β1, determined by Western blot analyses, was present in all progenitor cell preparations, as well as in erythrocytes. This finding is contrary to the expectation based on analysis of RNA by RT-PCR or Northern blots. Thus, the Na pump of human erythrocytes is composed of the isoforms 1 and 3 of the α subunit, isoforms 1, 2, and 3 of the β subunit, and a γ modulator.
It is important to understand that the findings presented in this work are interpreted to apply only to red blood cells and their lineage forms, and not to leukocytes or platelets (1). This distinction is based on the differences where the message for the β1 and β2 isoforms of the β subunit have been found in our preparations of RNA from human reticulocytes and erythroid progenitor cells. Analysis of erythroid-derived RNA showed that only the β2 but not the β1 isoform could be detected. In contrast, analysis of a leukocyte (and platelet containing) library showed the opposite, that only the β1 but not the β2 isoform could be observed (1). This difference, referred to herein as “β-profiling,” has been used as a routine screen in the present work to establish that our erythroid preparations are unlikely to be contaminated with white cell or platelet products.
The Na pump's functional properties are known to vary depending on the particular subunit combination of its isoforms, as well as the cellular/tissue environment in which these combinations are located (2–6). In addition, there are also variations in the pump's isoform composition that differ in their tissue/species distribution and in their developmental stage (2–4). Evidence regarding which Na pump isoforms are expressed in red blood cells is quite limited and variable: There are several reports that identify by Western blotting of human red cell ghosts the presence of the α1 isoform (7–10) and the β1 and β2 isoforms (9, 10), but not the α3 isoform (8–10); one group finds the α2 isoform present (9, 10), whereas another finds it absent (8). These previous results regarding the α2 and α3 isoform are not only inconsistent, but in direct conflict with the findings reported in this paper, and will be discussed later. The α1 isoform of the Na pump has also been seen in Western blots in red cell membranes obtained from horse, cow, pig, and a strain of high K (HK) containing dog red cells, but not in those from normal, low K (LK) dog or cat red cells (7). HK dog red cells were also shown by Western blotting to possess the β1 subunit, and reticulocytes from both HK and LK dogs express the α1 isoform that decreases on maturation in HK cells but is not discernable in LK cells (7). Messenger RNA obtained from the bone marrows of sheep displaying HK and LK red cell phenotypes were found to express the α1 and β1 isoforms, but not α2, α3, or β2 isoforms (11). Human bone marrow mRNA has been reported (1) to contain all of the Na pump isoforms except α4. The basis for this difference in isoform expression between sheep and humans is unknown. The γ modulator of the Na pump (2, 6, 12) has not been found in rat red cells (13), but the evidence presented below clearly documents, by both Northern and Western blot analyses, that the γ modulator is a normal constituent of precursor as well as mature human red blood cells. We have changed the notation to refer to the γ component as the γ modulator or FXYD2 rather than the γ subunit; a change that is consistent with its evident function as a member of the FXYD family (14). A preliminary account of this work has been presented (15).
Methods
Erythroid Progenitor Cells.
Human primary erythroid progenitor cells were derived by in vitro culture of CD34-positive cells isolated by A.W. from peripheral blood by modification of previously described methods (16–18). Growth factor mobilized peripheral blood from normal donors was purchased from All Cells, LLC (Berkeley, CA). These blood samples were separated over Ficoll/Hypaque (1.077 gm/ml; Amersham Pharmacia) to obtain mononuclear cells. CD34-positive cells were isolated from the mononuclear cells by using the antibody-coated paramagnetic microbeads in the CliniMACS cell isolation device (Miltenyi Biotec, Auburn, CA). The selected burst-forming-unit-erythroid (BFU-E) cells were >98% positive for CD34 as determined by flow cytometry. These were then cultured for 7–8 days as described (19) to obtain highly purified erythroid progenitors that were at the colony-forming-unit-erythroid stage of differentiation. The cell culture medium contained 15% FCS, 15% human AB serum, Iscove's modified Dulbecco's medium, 500 units/ml penicillin, 40 μg/ml streptomycin, 2 units/ml erythropoietin, 50 ng/ml stem cell factor, 10 ng/ml insulin-like growth factor-1, and 10 ng/ml interleukin-3. Greater than 90% of these cells were positive for CD71 (transferrin receptor) and glycophorin A, as determined by flow cytometry, and produced erythroid colonies when placed in methylcellulose.
These colony-forming-unit-erythroid cultures were further incubated from 7 to up to 14 days, during which time samples were removed for RNA and protein analysis as indicated. It should also be understood that during this incubation the cells differentiate, with 80–85% synchrony, through the various erythroid blast stages becoming orthochromatic erythroblasts/reticulocytes by day 14/15 (16, 17). The synthesis and expression of the major cytoskeletal proteins in these progenitor cells have been characterized during their maturation and terminal differentiation (17), where it is also evident that there is a discordance between the presence of membrane proteins and their respective mRNAs. The results presented are typical of the more than 14 separate individuals' cultured blood cells that were used in this and subsequent studies. In addition, all of the results reported were obtained in two or more separate sets of studies on different blood samples. RNA was extracted by using the RNeasy Midi kit (Qiagen, Valencia, CA). The extracted RNA was digested with DNase to remove any genomic DNA following the DNA-free kit protocol obtained from Ambion (Austin, TX). PCR was performed using PLATINUM Pfx DNA Polymerase from Cibro Invitrogen (Grand Island, NY) and following their procedure for PCR components and three-step cycling. PCR was conducted using a 96 Gradient Robocycler (Stratagene). The Na pump isoform specific primers used are given in Table 1.
Table 1.
PCR primer pair sequences that were selected to have distinct and high specificity for each of the α and β isoforms of the Na pump indicated
| Primer specificity | Expected product size, bp | Primer sequence |
|---|---|---|
| α1 | 530 | 5′-ATGGGGAAGGGGGTTGGACGTGATAA-3′ |
| 5′-TTCTCACCATTTCGAATCACAAGGGCTT-3′ | ||
| α2 | 610 | 5′-GGCGGCAAGAAGAAACAGAAGGAG-3′ |
| 5′-TGGGGCTCCGACTCTCCTGTTAAG-3′ | ||
| α3 | 518 | 5′-GACAAGAAAGATGACAAGGACTCACC-3′ |
| 5′-CCCGACCACCACCTCCTCAGCG-3′ | ||
| β1 | 441 | 5′-GGAAAGCCAAGGAGGAGGGCAGC-3′ |
| 5′-CTCCTCGTTCATGATTAAAGTCTCC-3′ | ||
| β2 | 455 | 5′-GTCCTCAACTACCCCAAACTGGCC-3′ |
| 5′-GTTGATGCGGAGTTTGAAGGCCAC-3′ |
Northern Blotting.
Northern blotting was carried out by following standard procedures (20). Total RNA, isolated from the progenitor cells, was electrophoresed in 1% agarose gels and blotted onto nylon membranes (GeneScreen, NEN Research Products, Boston). Each membrane was placed in 20 ml of prehybridization buffer (5× SSPE/50% formamide/1% SDS/5× Denhardt's solution/10% dextran sulfate/100 μg/ml denatured salmon sperm DNA; SSPE, 0.18 M NaCl/10 mM phosphate, pH 7.4/1 mM EDTA) for 3–4 h at 42°C, and then in 15 ml of hybridization buffer (6× SSPE/50% formamide/1% SDS/1× Denhardt's solution/10% dextran sulfate) containing denatured 32P-labeled DNA probe for 16–24 h. Membranes were washed in 2× SSPE at room temperature for 30 min, then in 2× SSPE/2% SDS at 42°C for 15 min and in 0.2× SSPE/0.1% SDS at 65°C for 30 min. After washing, membranes were exposed to Kodak XRP film at −70°C. For reblotting, membranes were washed two times in 0.1× SSPE/0.5% SDS at 95°C for 15 min.
DNA Probes.
Clones containing 3′ untranslated regions of all α and β isoforms of human Na/K pump cDNAs were ordered from WashU Merck EST Project, Washington University School of Medicine (St. Louis). PCR amplified fragments [as defined (1)] of the clones were used as specific probes for Northern blotting. The DNA fragments were radiolabeled by the random primary method using Random Priming DNA Labeling Kit and purified on G-50 Quick Spin Columns (Roche Molecular Biochemicals). For hybridization, 107 cpm of the labeled probe was used. Poly(A)+ RNA (#7760-1) of various human tissues was obtained from CLONTECH. These were used in the positive control blots for α2 and β1, as shown in Fig. 2.
Fig 2.
Northern blots of Na pump subunit isoforms present during maturation of cultured human erythroid progenitor cells (see Methods for details). Note that the α1 and α3 isoforms of the α subunit, as well as the β2 and β3 isoforms of the β subunit, are present from the 7th through the 14th day of differentiation. In contrast, the α2 isoform of the α subunit and the β1 isoform of the β subunit are absent during comparable time periods. Also depicted are the positive control blots of the α2 and β1 isoforms. H, heart; B, brain; P, placenta; L, lung; Li, liver; S, skeletal muscle; K, kidney; Pa, pancreas. These results parallel our previous findings (1) of the types of isoform messages present in human reticulocytes. And importantly, because the β2 isoform is present and the β1 isoform is absent, these results again emphasize that our erythroid progenitor cell cultures are essentially devoid of white cell or platelet contamination. Thus, the characteristics of β-profiling apply to the progenitor cell systems as used in this study.
Western Blotting.
For Western blot analysis, progenitor cells were lysed in 0.5% Triton X-100/150 mM NaCl/100 mM Na fluoride/1 mM EDTA/50 mM Hepes buffer/1.5 mM MgCl2/10 mM Na pyruvate/10% Glycerol/1 mM PMSF/10 μg/ml aprotinin. Protein concentrations were determined by Bio-Rad DC protein assay. Human brain and kidney protein medley (CLONTECH) were used as positive controls for detection of sodium pump isoforms. Proteins were resolved by electrophoresis through SDS/10% PAGE and transferred by electroblotting to Immobilon P membranes (Millipore). Each membrane was then blocked with 5% dry milk in TTBS [0.02 M Tris buffer, pH 5/0.l5 M NaCl/1 μl/ml Tween 20] for 1 h and incubated for another hour with the primary antibody. After incubation with the primary antibody, the membrane was washed with 5% dry milk in TTBS three times for 15 min before incubation for 1 h (1:1,000) with a secondary antibody [anti-mouse or anti-rabbit IgG antibody labeled with peroxidase (Sigma)]. Excess secondary antibody was removed by washing with TTBS three times for 15 min, and detection was performed according to the manufacturer's protocol (ECL Western Blotting, Amersham Pharmacia Biosciences).
Antibodies.
The following antibodies were used: C464.6, a mouse monoclonal against α1 (1:30,000) from M. Kashgarian (Yale University); McB2, a mouse monoclonal against α2 (1:500) from K. Sweadner (Harvard University, Boston); in addition, two rabbit polyclonals against α2 and α3 from M. Caplan (see ref. 21); Ma3-915, a mouse monoclonal against α3 (1:1,000), and Ma3-930, a monoclonal against β1 from Affinity Bioreagents (Golden, CO): SpET-β1, SpET-β2, and SpET-β3, rabbit polyclonals against β1, β2, and β3, respectively (1:20,000), from P. Martin-Vasallo (22, 23). Two different antibodies for the γ modulator were used: one, labeled M-Ab, from R. Mercer (Washington University) made to the N terminus (outside), and one, labeled B-Ab, from R. Blostein (McGill University, Montreal) made to the C terminus (inside) of the γ modulator.
Ouabain-Affinity Column: Purification of Na/K ATPase from Mature Human Erythrocytes.
Approximately 70 mg of human red cell membranes, prepared as described (24), was added to a stirred solution (≈40 ml) of 30 mM KCl, 1 mM EDTA, and 25 mM imidazole (pH 7.4) (K+ buffer). After the membranes were well mixed, ≈0.7 ml of 10% SDS was slowly added to a final concentration of 1.6 mg SDS/ml. After 30 min, ≈160 ml of the K+ buffer was added, followed by the addition of ≈2 ml of ouabain-affinity matrix (25). The resulting mixture (≈200 ml) was stirred for 1 hour at 4°C. The entire solution was poured into a column (25 mm diameter), the ouabain-affinity matrix was collected by filtration, and the resulting bed was washed at 4°C with ≈100 ml of the K+ buffer. After this solution was drained down to the top of the bed, the K+ buffer was washed off the ouabain affinity matrix by adding to the top of the column ≈0.5 ml of a solution containing 140 mM NaCl, 4 mM EDTA, 3 mM ATP, and 25 mM imidazole (pH 7.4) (elution buffer) while collecting an equal volume of buffer at the bottom of the column. Flow through the column was halted, ≈2 ml of elution buffer was added to the top of the bed, the column was put at 37°C, and the slurry gently mixed for 20 min. The column was then placed upright at room temperature, and ≈2 ml of elution buffer containing Na,K-ATPase was collected from the bottom of the column. This protein-containing sample was concentrated in a Centricon-100 filter system (Millipore) by centrifugation. This sample was then probed for the different Na pump isoforms and the γ modulator by Western blot analysis as described above.
Results
The first question we addressed was whether cultured human erythroid progenitor cells contained mRNA for the Na pump isoforms β1 and β2. We had previously found (1) with RNA extracted from purified human reticulocytes that the message for β2 was present, whereas that for β1 was absent. Thus, we performed RT-PCR on RNA extracted from erythroid progenitor cells by using the primers defined in Table 1. It is clear in the results presented in Fig. 1 that the β2 message was present in cells of all ages tested, but not that for β1 (1). Because white cells are known (1) to contain the β1 message, these results indicate that our progenitor cell preparations were free of contamination of nonerythroid cells. We call this difference in β1 and β2 mRNA content β-profiling and use this as an index for the purity of our preparations. The results in Fig. 1 also show the presence of mRNA for the α1 and α3 isoforms, but not that for α2, again paralleling the results found with reticulocyte RNA (1). It should be noted that the presence of α3 in the kidney control has been found before (26).
Fig 1.
Evidence for β-profiling in human erythroid progenitor cells. PCR products obtained with the isoform-specific primers as defined in Table 1. Single-stranded cDNA derived from erythroid progenitor cells or from samples of human kidney (K) or human bone marrow (BM) were used as template. It is evident that the β1 isoform is absent in erythroid cells 7, 9, or 12 days old, whereas β2 of the expected size (455 bp) is present. This result is consistent with our previous analysis of human reticulocyte RNA that shows what we refer to as β-profiling (1). β1 and β2 are both present in kidney at the expected sizes, respectively, of 441 and 455 bp. In addition, the α1 and α3 isoform products are both present in 7- and 12-day-old progenitor cells at the expected sizes, respectively, of 530 and 518 bp, together with these products in human kidney. In contrast, analysis for α2 in the same aged erythroid progenitor cells was absent (expected product size 610), whereas the bone marrow control showed this expected product size. The mass ladder in bp is on the left of each product group. There is an indication that the relative amounts of message present in these cells decreases with the culture time of incubation of the α1 and β2 isoforms, whereas that of α3 appears to be increasing.
The results of Northern blots of the various Na pump isoforms of human erythroid progenitor cells, cultured for different days, are presented in Fig. 2. It is evident that β-profiling is again displayed in this type of analysis. In addition, RNA for the Na pump isoforms α1 and α3 but not α2 are present together with β2 and β3. Analysis for the γ modulator RNA was not carried out in the erythroid progenitor cells, but it was found in reticulocyte RNA as reported (1). It should be noted that the levels of RNA for α1 and α3 isoforms diminish with time of incubation (Fig. 2), probably reflecting the normal loss of message as the cells differentiate and mature (see ref. 17). We have no explanation of why β1 message is degraded more rapidly than the other pump isoform messages, even though in our β1 instance the protein was made. Asynchrony between the loss of messages and the presence of red cell membrane proteins has been noted (17).
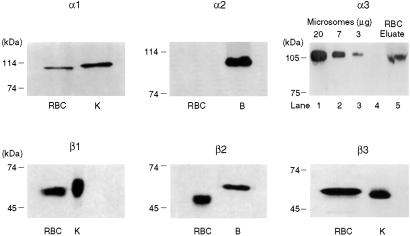
The next question concerned which Na pump proteins were expressed in erythroid progenitor cells and how they fared during their differentiation. To this end we solubilized the cells and carried out Western blotting, the results of which are shown in Fig. 3. Here it is clear that the β1 protein is present. This was contrary to the expectation based on RNA analysis (Figs. 1 and 2). In addition, the α1, α3, β2, and β3 isoforms are present but not the α2. And it is also clear that the γ modulator is expressed at the protein level in these progenitor cells.
Fig 3.
Western blots of Na pump isoforms present in the membranes of cultured human erythroid progenitor cells during their maturation from 7 to 14 days. Isoform controls are indicated as human brain (B) and kidney (K). The antibodies used together with other details are described in Methods. It is evident that in contrast to the RT-PCR and Northern blots presented in Figs. 1 and 2, respectively, the β1 isoform of the β subunit is present in these cells. Thus, these progenitor cells, incubated for 7 to up to 14 days, contain the α1 and α3 isoforms of the α subunit and the β1, β2, and β3 isoforms of the Na pump's β subunit. On the other hand, the α2 isoform appears to be absent in these cells. Control blots for all of the different isoforms are indicated with human brain (B) or human kidney (K). It is also apparent that the γ modulator of the Na pump is present in these progenitor cells, as evidenced by the use of two different antibodies, M-Ab and B-Ab, made to different regions of the γ modulator.
It was of special interest to study the Na pump protein composition in mature human erythrocytes. This was important not only to identify which Na pump isoforms were present in comparison to their maturation history, but also because all of the Na pump proteins reside in the ghost membrane. Because of the low pump density of human erythrocytes (about 400 per cell or 3 per μm2), a special procedure was devised to isolate and concentrate the Na pump proteins (25). The results of Western blots of Na pumps so isolated are presented in Figs. 4 and 5. In Fig. 4 it is evident that the results are completely comparable to those seen in the erythroid progenitor cells (Fig. 3). In addition, the results shown in Fig. 5 indicate that, with the use of two different antibodies, the γ modulator is also present. Thus, mature human erythrocytes, as well as the erythroid progenitor cells, contain the α1 and α3 isoforms of the α subunit, the β1, β2, and β3 isoforms of the β subunit, and the γ modulator. These results are summarized in Table 2.
Fig 4.
Western blots of Na pump isoforms present in the membranes of mature human erythrocytes (RBC). The Na pump was isolated, by means of a ouabain-linked agarose binding matrix, from solubilized human red cell ghost membranes as described in Methods. The Na pump was eluted from the binding matrix by exposure to a Mg2+-free solution that contained ATP, Na, and EDTA, the latter solution acting to decrease the affinity of the pump for ouabain as referenced in the text. The results shown here mirror completely the results presented in Fig. 3 of the isoform composition of progenitor cells. Thus, mature erythrocytes contain the α1 and α3 but not the α2 isoforms of the α subunit, as well as the β1, β2, and β3 isoforms of the β subunit. Control human tissue used in the blots are B for brain and K for kidney, except in the blot for α3, where rat brain microsomes were used.
Fig 5.
Western blots showing that the γ modulator is present in membranes of mature human erythrocytes. The methods used are the same as referred to in the legend of Fig. 4. The results are consistent with the detection of γ in erythroid progenitor cells as presented in Fig. 3, by using the same two antibodies as before. The left hand blots of the figure show that, with the M-Ab antibody, two bands appear on the left hand lane, with the lower band having the approximate same kDa as the positive control bands of γ expressed in SF9 cells. The middle lane is blank, indicating that no protein was obtained upon washing the column before the specific elution was executed. The right hand part of the figure shows the results obtained with the B-Ab antibody. In this instance, the positive controls for the γ subunit are rat kidney (Left) and SF9 cells (Right). In the Middle are the blots relative to the elution of the Na pump from mature human erythrocytes. LW, last wash; S/E, specific elution; SDS/E, elution by SDS. All of the blots at the level of the controls were exposed for 30 min. To image the γ modulator, the erythrocyte blot was further exposed overnight, with the result indicated by the arrow pointing to the lower box. The γ blot in this instance has a kDa the same as two control blots. The blots for the last red cell control wash and SDS were negative, indicating that no residual γ modulator was elutable. Because of uncertainties in extraction, absorption, elution, and blotting, we were unable to approximate the red cell content of γ or the ratio of α1 to γ.
Table 2.
Na,K-ATPase isoforms detected by Western analysis of human erythroid progenitor cells and mature human erythrocytes
| Cells | α1 | α2 | α3 | β1 | β2 | β3 | γ |
|---|---|---|---|---|---|---|---|
| Progenitors | + | − | + | + | + | + | + |
| Erythrocytes | + | − | + | + | + | + | + |
It is evident that the isoform composition of both types of preparations is the same, indicating that these cell types contain the α1, α3, β1, β2, and β3 isoforms, and the γ modulator of the Na pump.
Discussion
The results of the studies reported in this paper are summarized in Table 2. These findings, given the various antibodies that we used (see Methods), have been consistent and reproducible on different preparations of erythroid progenitor cells and mature erythrocytes. As mentioned before, results of others are in agreement with our finding that α1, β1, and β2 isoforms are present in red cell ghosts. On the other hand, reports concerning α2 and α3 are mixed. One group (9, 10) reports the presence of α2 but not α3 in the membranes of neonatal and mature erythrocytes. In contrast, we find α2 absent and α3 present. It is not clear that the antibodies used in the previous study (9, 10) were specific for these particular Na pump isoforms, and no data were presented concerning their cross-reactivity or characterization. The source of the antibodies they used is different from ours and is stated (27) to be from Upstate Biotechnology (Lake Placid, NY). The α2 and α3 antibodies that we used tested negatively for cross-reactivity, and their specificities are shown in the various blots. We used two different antibodies specific for α2 that gave the same results, and two different antibodies specific for α3 that also gave consistent results. The study reporting the absence of α2 (8) used the same antibody that we used (McB2); the fact that α3 was not found in that study (8) may relate to its low abundance in red cell ghosts (we estimate α3 to be ≈10–15% of α1). With regard to the γ modulator of the Na pump, the results of the Western blots presented in Figs. 3 and 5 show definitively that the γ modulator is a constituent component in the progenitor cells, and more importantly in mature human erythrocytes. By using the same antibody (B-Ab) in a much higher titer than used previously (13) in a study where the γ modulator was not detected, it was now clearly found (Fig. 5). The γ modulator was also seen in both progenitor cells (Fig. 3) and mature erythrocytes (Fig. 5) with a different antibody (M-Ab).
Assuming that we have established that the Na pump isoform composition of human red blood cells is as indicated above, a question arises about the significance of this mixture of the different isoforms. It is not known whether populations of red cells are homogenous or heterogeneous with regard to their Na pump isoform composition. Presumably, it would be possible to do in situ hybridization on single reticulocytes that could decide the issue. On the other hand, the transport characteristics of the Na pump as applied to red cells do not seem to be particularly sensitive to the isoform composition, except for the relative activity of α3 compared with α1 in combination with any of the three β isoforms. Previous work has shown (28) that the K1/2 values for internal Na and the turnover numbers are about the same for the combinations of α1β1, α1β2, and α1β3; both of these kinetic parameters are different for the α3 isoforms (see the Inset of Fig. 6) but with little variation seen among the combinations α3β1, α3β2, and α3β3. These various parameters were evaluated in Xenopus oocytes where all of the different combinations of human Na pump isoforms were expressed (28); similar type studies have also been carried out by others using different expression systems with either human (29) or rat (30, 31) Na pump isoforms where the differences in the kinetic parameters of α1 compared with α3 are similar to those given in the Inset of Fig. 6.
Fig 6.
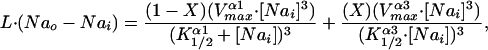 |
 and K
and K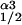 represent the relative affinities the two isoforms have for Nai as indicated in the inset together with their respective Vmax values. These kinetic parameters, determined at room temperature, are taken from ref. 28. It should also be noted that the turnover numbers given in table 1 (ref. 28) are taken to represent the Vmax values given in the Inset. The leak flux was determined by using Nai ≈ 7.0, Nao = 145, assuming only α1 present and the K1/2 and Vmax values given in the Inset. The leak value so obtained (0.025 sec−1) was assumed to remain constant independent of the ratio of α1 to α3. The values in the graph were obtained by choosing values of Nai and calculating X, keeping the total number of pumps, α1 + α3, constant. The denominators in the equation are raised to the power 3 to take into account that three sodium ions are effluxed per pump cycle. See text for discussion.
represent the relative affinities the two isoforms have for Nai as indicated in the inset together with their respective Vmax values. These kinetic parameters, determined at room temperature, are taken from ref. 28. It should also be noted that the turnover numbers given in table 1 (ref. 28) are taken to represent the Vmax values given in the Inset. The leak flux was determined by using Nai ≈ 7.0, Nao = 145, assuming only α1 present and the K1/2 and Vmax values given in the Inset. The leak value so obtained (0.025 sec−1) was assumed to remain constant independent of the ratio of α1 to α3. The values in the graph were obtained by choosing values of Nai and calculating X, keeping the total number of pumps, α1 + α3, constant. The denominators in the equation are raised to the power 3 to take into account that three sodium ions are effluxed per pump cycle. See text for discussion.In terms of how cells control the concentration of their intracellular Na (Nai), it was of interest to ask what the functional consequences might be for different combinations of α1 and α3, given just the differences in the values of their kinetic parameters, independent of any other regulatory factors. We approached this problem by assessing the steady-state concentration of Nai in a model system, such as a red blood cell, as a function of changing proportions of α1 and α3, holding the total number of pump units constant. The result is presented in Fig. 6, where it is evident that Nai increases as the ratio of α1 to α3 decreases. Even so, the steady-state value of Nai is relatively constant for up to a replacement of α1 by α3 by almost half, reflecting the differences in the kinetic parameters given in the Inset of Fig. 6. The shape of the curve shown is sensitive to the leak permeability of the membrane; the curve shifts upward (and steeper) with increases and downward (and flatter) with decreases in the leak value. We have yet to extend this analysis to include intracellular K or to evaluate any accompanying changes in cell volume. Because we estimate, qualitatively, from our various Western blots, that normal red cells contain 85–90% α1, it would appear that any α3 component would contribute minimally to the control of Nai.
There is evidence in a number of cell systems that the level of expression (i.e., number per unit area) of Na pumps in cell membranes is under the control of Nai, such that an increase in Nai leads to an increase in their expression (34, 35). This finding could represent an adaptative response to an increase in Nai by creating the circumstance to lower Nai by increased Na export. This type of homeostatic mechanism may be invoked to preserve cell volume. Another aspect of the mechanism that underlies this type of change in expression level rests in the dynamic state of Na pump synthesis/degradation and membrane turnover. But it is not clear what senses the Nai, how Na pump synthesis is initiated, and what control steps are involved in the regulation. More to the point of the present paper is: what controls the level of expression of the different Na pump isoforms that presumably occur in the same cell? In at least one instance, up-regulation appears to selectively increase the insertion of α2/α3- over α1-type isoforms in cultured cerebral neurons (36). But in red blood cells these events must take place during differentiation and maturation, recognizing that the Na pump density of the membrane, say of reticulocytes (which are devoid of intracellular pump components), is considerably increased over the number expressed in mature cells (see ref. 37 for references). The mechanism leading to the reduction of functioning Na pumps has yet to be defined.
Acknowledgments
We thank Drs. Cecilia Canessa, Michael Caplan, Maria Donoghue-Velleca, Rhoda Blostein, Patrick Gallagher, Edward Benz, Raymond Matting, and Katherine Massey for their considerable advice and help during the course of this work. We are also grateful to Michael Kashgarian, Kathleen Sweadner, Pablo Martin-Vasallo, Michael Caplan, Rhoda Blostein, and Robert Mercer for their gifts of antibodies; Michael Kashgarian for the sample of human kidney; and Maria Donoghue-Velleca for the sample of human brain. This work was supported in part by National Institutes of Health Grants HL 09906 (to J.F.H.), DK 37512 (to M.M.), and DK 60752 (to D.R.Y.), and by a grant from the Wendy Will Cay Cancer Fund (to A.W.).
References
- 1.Stengelin M. K. & Hoffman, J. F. (1997) Proc. Natl. Acad. Sci. USA 94, 5943-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco G. & Mercer, R. W. (1998) Am. J. Physiol. 275, F633-F650. [DOI] [PubMed] [Google Scholar]
- 3.Levenson R. (1994) Rev. Physiol. Biochem. Pharmacol. 123, 1-45. [DOI] [PubMed] [Google Scholar]
- 4.Lopez L. B., Quintas, L. E. M. & Noël, F. (2002) Comp. Biochem. Physiol. A Physiol. 131, 323-333. [DOI] [PubMed] [Google Scholar]
- 5.Therien A. G., Nestor, N. B., Ball, W. J. & Blostein, R. (1996) J. Biol. Chem. 271, 7104-7112. [DOI] [PubMed] [Google Scholar]
- 6.Therien A. G. & Blostein, R. (2000) Am. J. Physiol. 279, C541-C566. [DOI] [PubMed] [Google Scholar]
- 7.Inaba M. & Maede, Y. (1986) J. Biol. Chem. 261, 16099-16105. [PubMed] [Google Scholar]
- 8.McDonough A. A., Wang, J. & Farley, R. A. (1995) J. Mol. Cell. Cardiol. 27, 1001-1009. [DOI] [PubMed] [Google Scholar]
- 9.Vásárhelyi B., Vér, Á., Nobilis, A., Szabó, T. & Tulassay, T. (1998) Eur. J. Clin. Invest. 28, 543-545. [DOI] [PubMed] [Google Scholar]
- 10.Vásárhelyi B., Tulassay, T., Vér, Á., Dobos, M., Kocsis, I. & Seri, I. (2000) Arch. Dis. Child. Fetal Neonatal Ed. 83, F135-F138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhir R., Nishioka, Y. & Blostein, R. (1990) Biochim. Biophys. Acta 1026, 141-146. [DOI] [PubMed] [Google Scholar]
- 12.Forbush B., Kaplan, J. H. & Hoffman, J. F. (1978) Biochemistry 17, 3667-3676. [DOI] [PubMed] [Google Scholar]
- 13.Therien A. G., Goldshleger, R., Karlish, S. J. D. & Blostein, R. (1997) J. Biol. Chem. 272, 32628-32634. [DOI] [PubMed] [Google Scholar]
- 14.Sweadner K. J. & Rael, E. (2000) Genomics 68, 41-56. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman J. F., Potapova, O., Wickrema, A. & Yingst, D. R. (2000) in Na/K-ATPase and Related ATPases, eds. Taniguchi, K. & Kaya, W. (Elsevier, New York), pp. 449–450.
- 16.Wickrema A., Krantz, S. B., Winkelmann, J. C. & Bondurant, M. D. (1992) Blood 80, 1940-1949. [PubMed] [Google Scholar]
- 17.Wickrema A., Koury, S. T., Dai, C.-H. & Krantz, S. B. (1994) J. Cell. Physiol. 160, 417-426. [DOI] [PubMed] [Google Scholar]
- 18.Wickrema A., Uddin, S., Sharma, A., Chen, F., Alsayed, Y., Ahmad, S., Sawyer, S. T., Krystal, G., Yi, T., Nishada, K., Hibi, M., et al. (1999) J. Biol. Chem. 274, 24469-24474. [DOI] [PubMed] [Google Scholar]
- 19.Mahmud D. L., Amlak, M.-G., Deb, D. K., Platanias, L. C., Uddin, S. & Wickrema, A. (2002) Oncogene 21, 1556-1562. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J., Fritsch, E. F. & Maniatis, T., (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 21.Pietrini G., Matteoli, M., Banker, G. & Caplan, M. J. (1992) Proc. Natl. Acad. Sci. USA 89, 8414-8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Martinez L. M., Avila, J., Martí, E., Lecuona, E. & Martín-Vasallo, P. (1994) Biol. Cell 81, 215-222. [DOI] [PubMed] [Google Scholar]
- 23.Avila J., Alvarez de la Rosa, D., González-Martinez, L. M., Lecuona, E. & Martín-Vasallo, P. (1998) Gene 208, 221-227. [DOI] [PubMed] [Google Scholar]
- 24.Yingst D. R., Ye-Hu, J., Chen, H. & Barrett, V. (1992) Arch. Biochem. Biophys. 295, 49-54. [DOI] [PubMed] [Google Scholar]
- 25.Yingst D. R., Yang, S.-Y. & Schiebinger, R. (1998) Am. J. Physiol. 275, C1167-C1177. [DOI] [PubMed] [Google Scholar]
- 26.Lucking K., Nielsen, J. M., Pedersen, P. A. & Jorgensen, P. L. (1996) Am. J. Physiol. 271, F253-F260. [DOI] [PubMed] [Google Scholar]
- 27.Vér A., Csermely, P., Bányász, T., Kovács, T. & Somogyi, J. (1995) Biochim. Biophys. Acta 1237, 143-150. [DOI] [PubMed] [Google Scholar]
- 28.Crambert G., Hasler, U., Beggah, A. T., Yu, C., Modyanov, N. N., Horisberger, J.-D., Leliévre, L. & Geering, K. (2000) J. Biol. Chem. 275, 1976-1986. [DOI] [PubMed] [Google Scholar]
- 29.Müller-Ehmsen J., Juvvadi, P., Thompson, C. B., Tumyan, L., Croyle, M., Lingrel, J. B., Schwinger, R. H. G., McDonough, A. A. & Farley, R. A. (2001) Am. J. Physiol. 281, C1355-C1364. [DOI] [PubMed] [Google Scholar]
- 30.Zahler R., Zhang, Z.-T., Manor, M. & Boron, W. (1997) J. Gen. Physiol. 110, 201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segall L., Daly, S. E. & Blostein, R. (2001) J. Biol. Chem. 276, 31535-31541. [DOI] [PubMed] [Google Scholar]
- 32.Tosteson D. C. & Hoffman, J. F. (1960) J. Gen. Physiol. 44, 169-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milanick M. A. & Hoffman, J. F. (1986) Ann. N.Y. Acad. Sci. 488, 174-186. [DOI] [PubMed] [Google Scholar]
- 34.Boardman L., Huett, M., Lamb, J. F., Newton, J. P. & Polson, J. M. (1974) J. Physiol. 241, 771-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollack L. R., Tate, E. H. & Cook, J. S. (1981) Am. J. Physiol. 241, C173-C183. [DOI] [PubMed] [Google Scholar]
- 36.Inoue N., Soga, T. & Kato, T. (1999) NeuroReport 10, 3289-3293. [DOI] [PubMed] [Google Scholar]
- 37.Mairbäurl H., Schulz, S. & Hoffman, J. F. (2000) Am. J. Physiol. 279, C1621-C1630. [DOI] [PubMed] [Google Scholar]