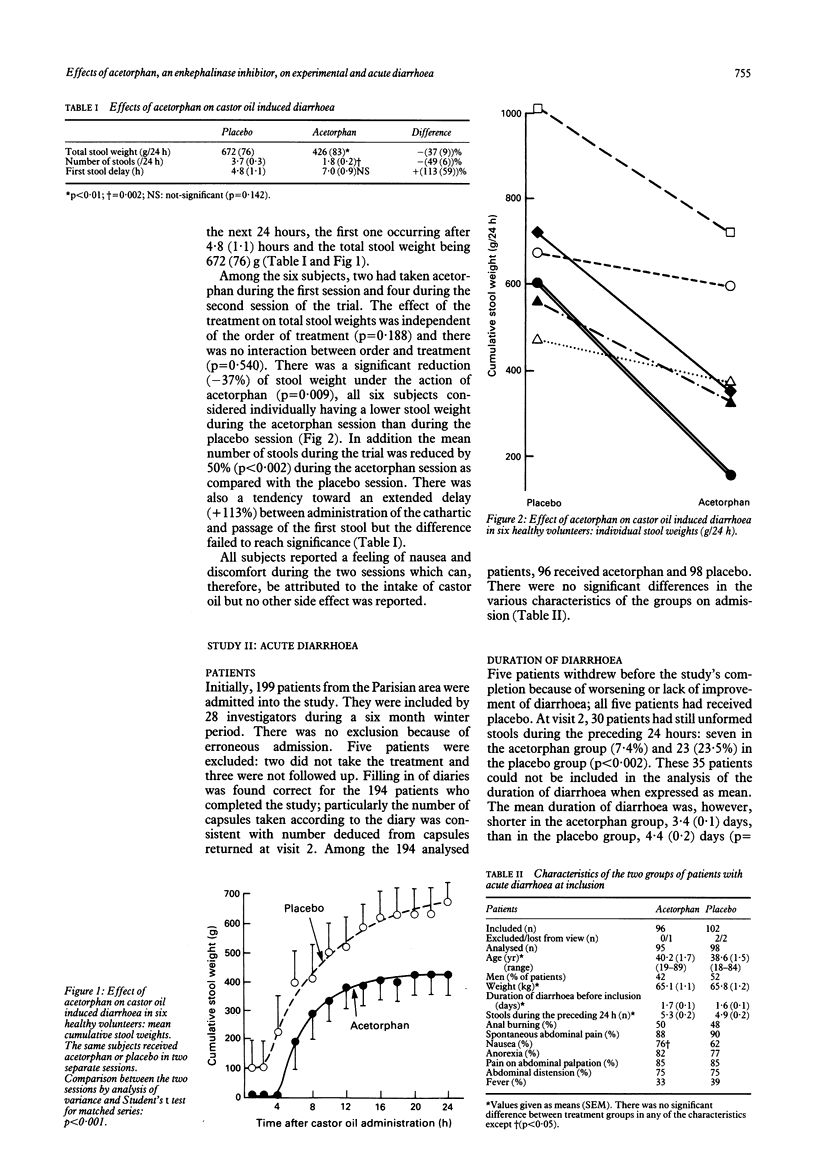
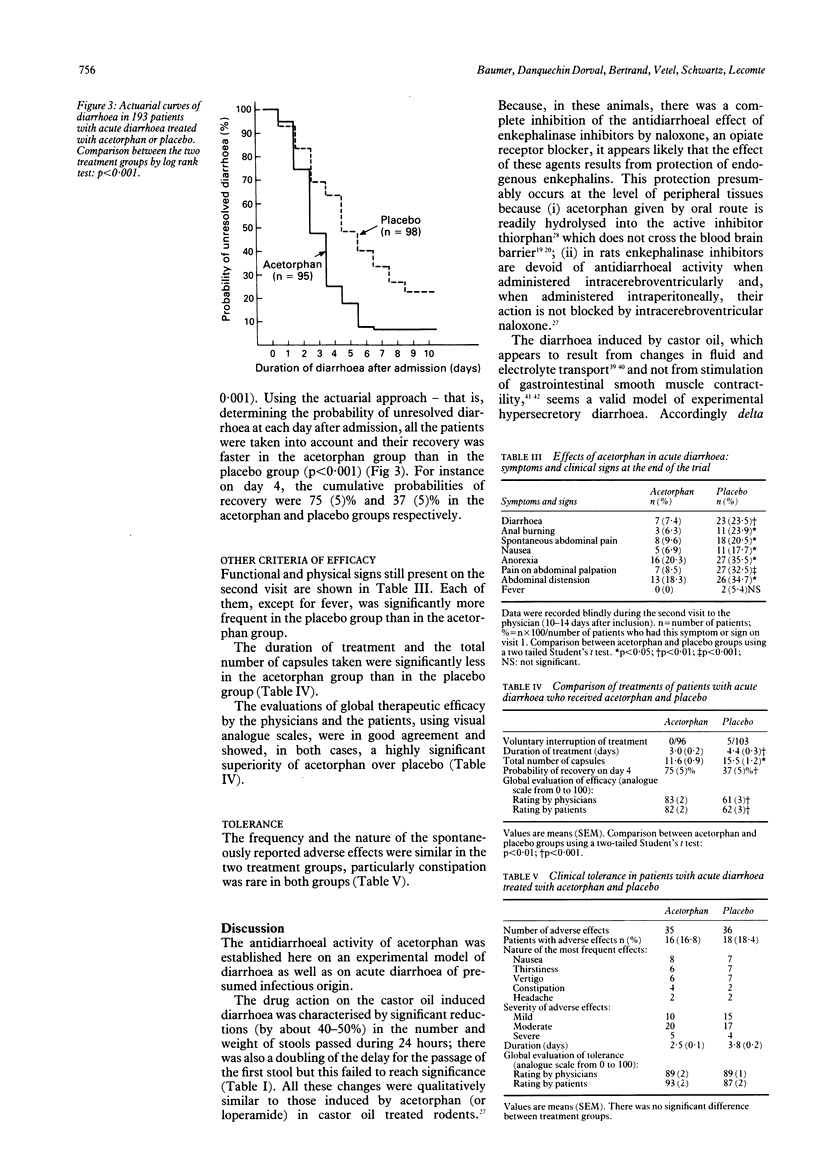
Abstract
Acetorphan is an orally active inhibitor of enkephalinase (EC 3.4.24.11) with antidiarrhoeal activity in rodents apparently through protection of endogenous enkephalins and a purely antisecretory mechanism. Its antidiarrhoeal activity in man was assessed in an experimental model of cathartic induced secretory diarrhoea as well as in acute diarrhoea of presumed infectious origin. In six healthy volunteers receiving castor oil and pretreated with acetorphan or placebo in a crossover controlled trial, the drug significantly decreased the number and weight of stools passed during 24 hours. About 200 outpatients with severe acute diarrhoea (more than five stools per day) were included in a randomised double blind study of acetorphan against placebo. The significant antidiarrhoeal activity of acetorphan was established using a variety of criteria: (i) the duration of both diarrhoea and treatment were diminished; (ii) no acetorphan treated patient withdrew from the study whereas five dropped out because of worsening in the placebo group; (iii) the frequency of symptoms associated with diarrhoea--for example, abdominal pain or distension, nausea and anorexia--remaining after two weeks was nearly halved; (iv) using visual analogue scales acetorphan treatment was found more effective than placebo by both investigators and patients. There was statistically no significant difference between acetorphan and placebo in respect of side effects, particularly constipation, which often accompanies the antidiarrhoeal activity of mu opioid receptor agonists this difference is attributable to the lack of antipropulsive activity of acetorphan in man. The efficacy and tolerance of acetorphan suggest that enkephalinase inhibition may represent a novel therapeutic approach for the symptomatic management of acute secretory diarrhoea without impairing intestinal transit.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. V., Thomas P. J., Phillips S. F. Effects of oleic and ricinoleic acids on net jejunal water and electrolyte movement. Perfusion studies in man. J Clin Invest. 1974 Feb;53(2):374–379. doi: 10.1172/JCI107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K., Kajiwara M., Oka T. The role of bestatin-sensitive aminopeptidase, angiotensin converting enzyme and thiorphan-sensitive "enkephalinase" in the potency of enkephalins in the guinea-pig ileum. Jpn J Pharmacol. 1984 Sep;36(1):59–65. doi: 10.1254/jjp.36.59. [DOI] [PubMed] [Google Scholar]
- Basilisco G., Bozzani A., Camboni G., Recchia M., Quatrini M., Conte D., Penagini R., Bianchi P. A. Effect of loperamide and naloxone on mouth-to-caecum transit time evaluated by lactulose hydrogen breath test. Gut. 1985 Jul;26(7):700–703. doi: 10.1136/gut.26.7.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilisco G., Camboni G., Bozzani A., Paravicini M., Bianchi P. A. Oral naloxone antagonizes loperamide-induced delay of orocecal transit. Dig Dis Sci. 1987 Aug;32(8):829–832. doi: 10.1007/BF01296704. [DOI] [PubMed] [Google Scholar]
- Berschneider H. M., Martens H., Powell D. W. Effect of BW 942C, an enkephalinlike pentapeptide, on sodium and chloride transport in the rabbit ileum. Gastroenterology. 1988 Jan;94(1):127–136. doi: 10.1016/0016-5085(88)90620-8. [DOI] [PubMed] [Google Scholar]
- Bright-Asare P., Binder H. J. Stimulation of colonic secretion of water and electrolytes by hydroxy fatty acids. Gastroenterology. 1973 Jan;64(1):81–88. [PubMed] [Google Scholar]
- Brown J. W. Toxic megacolon associated with loperamide therapy. JAMA. 1979 Feb 2;241(5):501–502. [PubMed] [Google Scholar]
- Chipkin R. E., Berger J. G., Billard W., Iorio L. C., Chapman R., Barnett A. Pharmacology of SCH 34826, an orally active enkephalinase inhibitor analgesic. J Pharmacol Exp Ther. 1988 Jun;245(3):829–838. [PubMed] [Google Scholar]
- Dobbins J., Racusen L., Binder H. J. Effect of D-alanine methionine enkephalin amide on ion transport in rabbit ileum. J Clin Invest. 1980 Jul;66(1):19–28. doi: 10.1172/JCI109830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pont H. L. Nonfluid therapy and selected chemoprophylaxis of acute diarrhea. Am J Med. 1985 Jun 28;78(6B):81–90. doi: 10.1016/0002-9343(85)90369-9. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B. Adverse effect of lomotil therapy in shigellosis. JAMA. 1973 Dec 24;226(13):1525–1528. [PubMed] [Google Scholar]
- Edelman R. Prevention and treatment of infectious diarrhea. Speculations on the next 10 years. Am J Med. 1985 Jun 28;78(6B):99–106. doi: 10.1016/0002-9343(85)90371-7. [DOI] [PubMed] [Google Scholar]
- Gaginella T. S., Stewart J. J., Olsen W. A., Bass P. Actions of ricinoleic acid and structurally related fatty acids on the gastrointestinal tract. II. Effects on water and electrolyte absorption in vitro. J Pharmacol Exp Ther. 1975 Nov;195(2):355–361. [PubMed] [Google Scholar]
- Garrett J. M., Sauer W. G., Moertel C. G. Colonic motility in ulcerative colitis after opiate administration. Gastroenterology. 1967 Jul;53(1):93–100. [PubMed] [Google Scholar]
- Jivegård L., Pollard H., Moreau J., Schwartz J. C., Thune A., Svanvik J. Naloxone-reversible inhibition of gall-bladder mucosal fluid secretion in experimental cholecystitis in the cat by acetorphan, an enkephalinase inhibitor. Clin Sci (Lond) 1989 Jul;77(1):49–54. doi: 10.1042/cs0770049. [DOI] [PubMed] [Google Scholar]
- Kachel G., Ruppin H., Hagel J., Barina W., Meinhardt M., Domschke W. Human intestinal motor activity and transport: effects of a synthetic opiate. Gastroenterology. 1986 Jan;90(1):85–93. doi: 10.1016/0016-5085(86)90079-x. [DOI] [PubMed] [Google Scholar]
- Kachur J. F., Miller R. J. Characterization of the opiate receptor in the guinea-pig ileal mucosa. Eur J Pharmacol. 1982 Jul 9;81(2):177–183. doi: 10.1016/0014-2999(82)90435-6. [DOI] [PubMed] [Google Scholar]
- Kachur J. F., Miller R. J., Field M. Control of guinea pig intestinal electrolyte secretion by a delta-opiate receptor. Proc Natl Acad Sci U S A. 1980 May;77(5):2753–2756. doi: 10.1073/pnas.77.5.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely J. S., Beardsley P. M., Aceto M. D., Balster R. L., Harris L. S. Assessment of the abuse potential of acetorphan, an enkephalinase inhibitor. Drug Alcohol Depend. 1989 Apr;23(2):143–151. doi: 10.1016/0376-8716(89)90020-3. [DOI] [PubMed] [Google Scholar]
- Lecomte J. M., Costentin J., Vlaiculescu A., Chaillet P., Marcais-Collado H., Llorens-Cortes C., Leboyer M., Schwartz J. C. Pharmacological properties of acetorphan, a parenterally active "enkephalinase" inhibitor. J Pharmacol Exp Ther. 1986 Jun;237(3):937–944. [PubMed] [Google Scholar]
- Llorens C., Schwartz J. C. Enkephalinase activity in rat peripheral organs. Eur J Pharmacol. 1981 Jan 5;69(1):113–116. doi: 10.1016/0014-2999(81)90609-9. [DOI] [PubMed] [Google Scholar]
- Marcovitch H. Loperamide in "toddler diarrhoea". Lancet. 1980 Jun 28;1(8183):1413–1413. doi: 10.1016/s0140-6736(80)92673-2. [DOI] [PubMed] [Google Scholar]
- Marçais-Collado H., Uchida G., Costentin J., Schwartz J. C., Lecomte J. M. Naloxone-reversible antidiarrheal effects of enkephalinase inhibitors. Eur J Pharmacol. 1987 Dec 1;144(2):125–132. doi: 10.1016/0014-2999(87)90510-3. [DOI] [PubMed] [Google Scholar]
- McArthur K. E., Anderson D. S., Durbin T. E., Orloff M. J., Dharmsathaphorn K. Clonidine and lidamidine to inhibit watery diarrhea in a patient with lung cancer. Ann Intern Med. 1982 Mar;96(3):323–325. doi: 10.7326/0003-4819-96-3-323. [DOI] [PubMed] [Google Scholar]
- McKelvy J. F., Blumberg S. Inactivation and metabolism of neuropeptides. Annu Rev Neurosci. 1986;9:415–434. doi: 10.1146/annurev.ne.09.030186.002215. [DOI] [PubMed] [Google Scholar]
- McKnight A. T., Corbett A. D., Kosterlitz H. W. Increase in potencies of opioid peptides after peptidase inhibition. Eur J Pharmacol. 1983 Jan 21;86(3-4):393–402. doi: 10.1016/0014-2999(83)90189-9. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986 Jun;88(2):315–322. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak E., Lee J. G., Seckman C. E., Phillips J. P., DiSanto A. R. Unfavorable effect of atropine-diphenoxylate (Lomotil) therapy in lincomycin-caused diarrhea. JAMA. 1976 Apr 5;235(14):1451–1454. [PubMed] [Google Scholar]
- O'Brien J. D., Thompson D. G., McIntyre A., Burnham W. R., Walker E. Effect of codeine and loperamide on upper intestinal transit and absorption in normal subjects and patients with postvagotomy diarrhoea. Gut. 1988 Mar;29(3):312–318. doi: 10.1136/gut.29.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H., Moreau J., Ronco P., Verroust P., Schwartz J. C. Immunoautoradiographic localisation of enkephalinase (EC 3.4.24.11) in rat gastrointestinal tract. Neuropeptides. 1991 Jul;19(3):169–178. doi: 10.1016/0143-4179(91)90115-y. [DOI] [PubMed] [Google Scholar]
- Powell D. W. Muscle or mucosa: the site of action of antidiarrheal opiates? Gastroenterology. 1981 Feb;80(2):406–408. [PubMed] [Google Scholar]
- Pressman J. H., Hofmann A. F., Witztum K. F., Gertler S. L., Steinbach J. H., Stokes K., Kelts D. G., Stone D. M., Jones B. R., Dharmsathaphorn K. Limitations of indirect methods of estimating small bowel transit in man. Dig Dis Sci. 1987 Jul;32(7):689–699. doi: 10.1007/BF01296133. [DOI] [PubMed] [Google Scholar]
- Ruppin H. Review: loperamide--a potent antidiarrhoeal drug with actions along the alimentary tract. Aliment Pharmacol Ther. 1987 Jun;1(3):179–190. doi: 10.1111/j.1365-2036.1987.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Ryan J., Leighton J., Kirksey D., McMahon G. Evaluation of an enkephalin analog in men with castor oil-induced diarrhea. Clin Pharmacol Ther. 1986 Jan;39(1):40–42. doi: 10.1038/clpt.1986.7. [DOI] [PubMed] [Google Scholar]
- Schiller L. R., Davis G. R., Santa Ana C. A., Morawski S. G., Fordtran J. S. Studies of the mechanism of the antidiarrheal effect of codeine. J Clin Invest. 1982 Nov;70(5):999–1008. doi: 10.1172/JCI110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller L. R., Santa Ana C. A., Morawski S. G., Fordtran J. S. Mechanism of the antidiarrheal effect of loperamide. Gastroenterology. 1984 Jun;86(6):1475–1480. [PubMed] [Google Scholar]
- Schuermans V., Van Lommel R., Dom J., Brugmans J. Loperamide (R 18 553), a novel type of antidiarrheal agent. Part 6: Clinical pharmacology. Placebo-controlled comparison of the constipating activity and safety of loperamide, diphenoxylate and codeine in normal volunteers. Arzneimittelforschung. 1974 Oct;24(10):1653–1657. [PubMed] [Google Scholar]
- Shook J. E., Lemcke P. K., Gehrig C. A., Hruby V. J., Burks T. F. Antidiarrheal properties of supraspinal mu and delta and peripheral mu, delta and kappa opioid receptors: inhibition of diarrhea without constipation. J Pharmacol Exp Ther. 1989 Apr;249(1):83–90. [PubMed] [Google Scholar]
- Sninsky C. A., Davis R. H., Clench M. H., Thomas K. D., Mathias J. R. Effect of lidamidine hydrochloride and loperamide on gastric emptying and transit of the small intestine. A double-blind study. Gastroenterology. 1986 Jan;90(1):68–73. doi: 10.1016/0016-5085(86)90076-4. [DOI] [PubMed] [Google Scholar]
- Staniforth D. H. Effect of drugs on oro-caecal transit time assessed by the lactulose/breath hydrogen method. Eur J Clin Pharmacol. 1987;33(1):55–58. doi: 10.1007/BF00610380. [DOI] [PubMed] [Google Scholar]
- Stewart J. J., Gaginella T. S., Bass P. Actions of ricinoleic acid and structurally related fatty acids of the gastrointestinal tract. I. Effects on smooth muscle contractility in vitro. J Pharmacol Exp Ther. 1975 Nov;195(2):347–354. [PubMed] [Google Scholar]
- van Amsterdam J. G., van Buuren K. J., Krielaart M. J., Zuiderveld O. P., Tijms R. P. Effect of inhibitors of enkephalin degradation in the isolated guinea-pig ileum. Life Sci. 1988;43(19):1529–1536. doi: 10.1016/0024-3205(88)90401-8. [DOI] [PubMed] [Google Scholar]


