Abstract
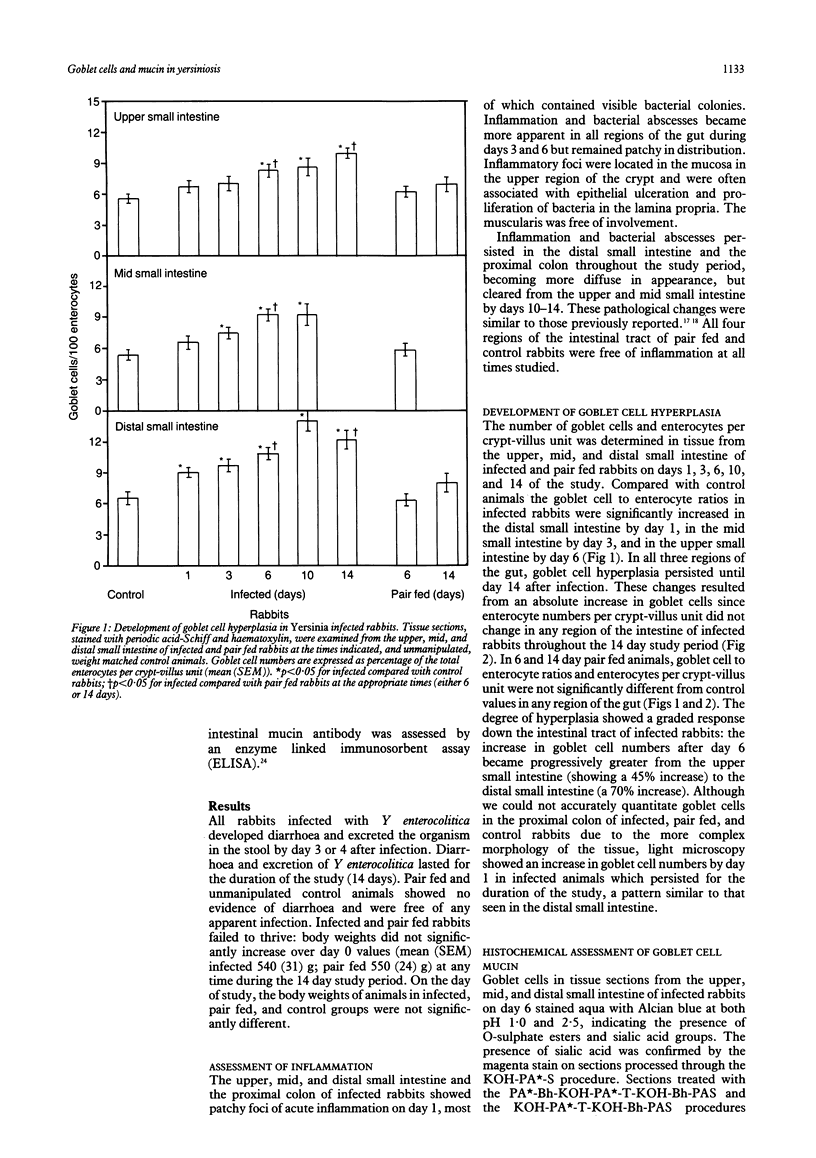
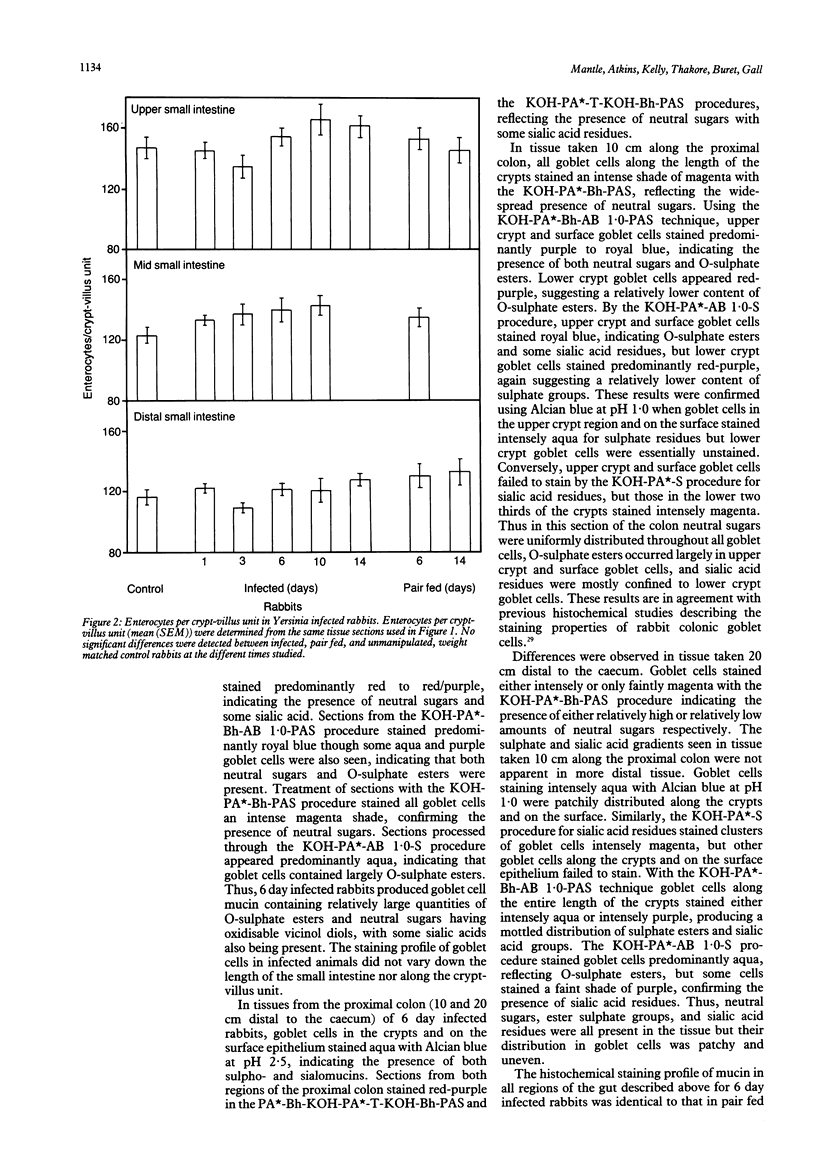
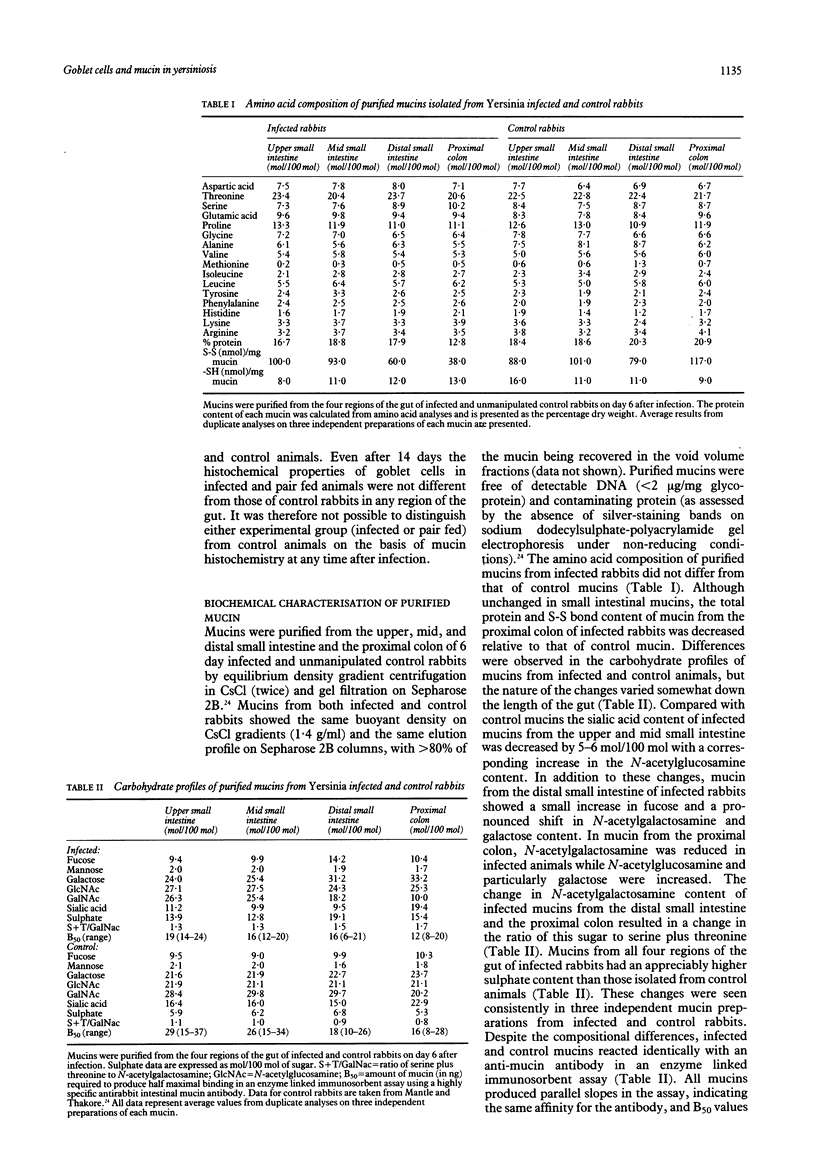
The effects of Yersinia enterocolitica on intestinal goblet cells were investigated in New Zealand white rabbits. Animals infected with Y enterocolitica were compared with weight matched and pair fed controls. Goblet cell hyperplasia developed in the distal small intestine of infected rabbits on day 1, in the mid small intestine on day 3, and in the upper small intestine on day 6. In all regions hyperplasia persisted throughout the 14 day study. The degree of hyperplasia was greater in the distal small intestine than the upper and mid regions. Goblet cells in the proximal colon of infected animals seemed to respond as those in the distal small intestine. Thus goblet cell hyperplasia developed more rapidly and to a greater extent in the ileocaecal region where mucosal injury was most severe. These changes resulted directly from Y enterocolitica infection since goblet cell numbers did not increase in pair fed controls. Histochemically, goblet cell mucins from infected rabbits were unchanged at either six or 14 days. Biochemical analysis, however, established that purified mucins from animals on day 6 after infection were less sialylated (in the small intestine) and more sulphated (in the small intestine and proximal colon). In addition, mucins from the distal small intestine and the proximal colon seemed to contain fewer but longer oligosaccharide chains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buret A., O'Loughlin E. V., Curtis G. H., Gall D. G. Effect of acute Yersinia enterocolitica infection on small intestinal ultrastructure. Gastroenterology. 1990 Jun;98(6):1401–1407. doi: 10.1016/0016-5085(90)91068-h. [DOI] [PubMed] [Google Scholar]
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Cope G. F., Heatley R. V., Kelleher J., Axon A. T. In vitro mucus glycoprotein production by colonic tissue from patients with ulcerative colitis. Gut. 1988 Feb;29(2):229–234. doi: 10.1136/gut.29.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanullah M., Filipe M. I., Gazzard B. Morphological and mucus secretion criteria for differential diagnosis of solitary ulcer syndrome and non-specific proctitis. J Clin Pathol. 1982 Jan;35(1):26–30. doi: 10.1136/jcp.35.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanullah M., Filipe M. I., Gazzard B. Mucin secretion in inflammatory bowel disease: correlation with disease activity and dysplasia. Gut. 1982 Jun;23(6):485–489. doi: 10.1136/gut.23.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I., Fenger C. Histochemical characteristics of mucins in the small intestine. A comparative study of normal mucosa, benign epithelial tumours and carcinoma. Histochem J. 1979 May;11(3):277–287. doi: 10.1007/BF01005027. [DOI] [PubMed] [Google Scholar]
- Forstner J., Maxwell B., Roomi N. Intestinal secretion of mucin in chronically reserpine-treated rats. Am J Physiol. 1981 Nov;241(5):G443–G450. doi: 10.1152/ajpgi.1981.241.5.G443. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Koninkx J. F., Mirck M. H., Hendriks H. G., Mouwen J. M., van Dijk J. E. Nippostrongylus brasiliensis: histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp Parasitol. 1988 Feb;65(1):84–90. doi: 10.1016/0014-4894(88)90109-9. [DOI] [PubMed] [Google Scholar]
- Mantle M., Stewart G. Intestinal mucins from normal subjects and patients with cystic fibrosis. Variable contents of the disulphide-bound 118 kDa glycoprotein and different reactivities with an anti-(118 kDa glycoprotein) antibody. Biochem J. 1989 Apr 1;259(1):243–253. doi: 10.1042/bj2590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Stewart G., Zayas G., King M. The disulphide-bond content and rheological properties of intestinal mucins from normal subjects and patients with cystic fibrosis. Biochem J. 1990 Mar 1;266(2):597–604. [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Thakore E., Hardin J., Gall D. G. Effect of Yersinia enterocolitica on intestinal mucin secretion. Am J Physiol. 1989 Feb;256(2 Pt 1):G319–G327. doi: 10.1152/ajpgi.1989.256.2.G319. [DOI] [PubMed] [Google Scholar]
- Mantle M., Thakore E. Rabbit intestinal and colonic mucins: isolation, partial characterization, and measurement of secretion using an enzyme-linked immunoassay. Biochem Cell Biol. 1988 Oct;66(10):1045–1054. doi: 10.1139/o88-121. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Nawa Y. Nippostrongylus brasiliensis: intestinal goblet-cell response in adoptively immunized rats. Exp Parasitol. 1979 Feb;47(1):81–90. doi: 10.1016/0014-4894(79)90010-9. [DOI] [PubMed] [Google Scholar]
- Morrissey S. M., Tymvios M. C. Acid mucins in human intestinal goblet cells. J Pathol. 1978 Dec;126(4):197–208. doi: 10.1002/path.1711260403. [DOI] [PubMed] [Google Scholar]
- Morrissey S. M., Ward P. M., Jayaraj A. P., Tovey F. I., Clark C. G. Histochemical changes in mucus in duodenal ulceration. Gut. 1983 Oct;24(10):909–913. doi: 10.1136/gut.24.10.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Loughlin E. V., Humphreys G., Dunn I., Kelly J., Lian C. J., Pai C., Gall D. G. Clinical, morphological, and biochemical alterations in acute intestinal Yersiniosis. Pediatr Res. 1986 Jul;20(7):602–608. doi: 10.1203/00006450-198607000-00005. [DOI] [PubMed] [Google Scholar]
- O'Loughlin E. V., Pai C. H., Gall D. G. Effect of acute Yersinia enterocolitica infection on in vivo and in vitro small intestinal solute and fluid absorption in the rabbit. Gastroenterology. 1988 Mar;94(3):664–672. doi: 10.1016/0016-5085(88)90237-5. [DOI] [PubMed] [Google Scholar]
- O'Loughlin E. V., Pai C. H., Hardin J. A., Gall D. G. Colonic function in acute Yersinia enterocolitica infection in rabbits. Clin Invest Med. 1988 Oct;11(5):366–372. [PubMed] [Google Scholar]
- Pai C. H., Mors V., Seemayer T. A. Experimental Yersinia enterocolitica enteritis in rabbits. Infect Immun. 1980 Apr;28(1):238–244. doi: 10.1128/iai.28.1.238-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. E., Culling C. F., Dunn W. L., Clay M. G. Chemical and histochemical studies of normal and diseased human gastrointestinal tract. II. A comparison between histologically normal small intestine and Crohn's disease of the small intestine. Histochem J. 1984 Mar;16(3):253–264. doi: 10.1007/BF01003609. [DOI] [PubMed] [Google Scholar]
- Reid P. E., Owen D. A., Dunn W. L., Ramey C. W., Lazosky D. A., Clay M. G. Chemical and histochemical studies of normal and diseased human gastrointestinal tract. III. Changes in the histochemical and chemical properties of the epithelial glycoproteins in the mucosa close to colonic tumours. Histochem J. 1985 Feb;17(2):171–181. doi: 10.1007/BF01003216. [DOI] [PubMed] [Google Scholar]
- Reid P. E., Walker D. C., Terpin T., Owen D. A. Histochemical studies of the colonic epithelial glycoproteins of the normal rabbit. Histochem J. 1988 Oct;20(10):533–550. doi: 10.1007/BF01002608. [DOI] [PubMed] [Google Scholar]
- Silvestri L. J., Hurst R. E., Simpson L., Settine J. M. Analysis of sulfate in complex carbohydrates. Anal Biochem. 1982 Jul 1;123(2):303–309. doi: 10.1016/0003-2697(82)90450-x. [DOI] [PubMed] [Google Scholar]
- Volz D., Reid P. E., Park C. M., Owen D. A., Dunn W. L. A new histochemical method for the selective periodate oxidation of total tissue sialic acids. Histochem J. 1987 Jun-Jul;19(6-7):311–318. doi: 10.1007/BF01680446. [DOI] [PubMed] [Google Scholar]
- Volz D., Reid P. E., Park C. M., Owen D. A., Dunn W. L. Histochemical procedures for the simultaneous visualization of neutral sugars and either sialic acid and its side chain O-acyl variants or O-sulphate ester. I. Methods based upon the selective periodate oxidation of sialic acids. Histochem J. 1987 May;19(5):249–256. doi: 10.1007/BF01675683. [DOI] [PubMed] [Google Scholar]
- Wesley A., Forstner J., Qureshi R., Mantle M., Forstner G. Human intestinal mucin in cystic fibrosis. Pediatr Res. 1983 Jan;17(1):65–69. doi: 10.1203/00006450-198301000-00013. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. The limitations of immunoenzyme approaches to distinguish between 'specific' and 'non-specific' antibody-forming cells, with particular respect to immunocytochemical studies on the in situ immune response. Histochem J. 1987 Jun-Jul;19(6-7):369–374. doi: 10.1007/BF01680454. [DOI] [PubMed] [Google Scholar]


