Abstract
1 The long term safety and efficacy of vigabatrin has been studied in 254 patients with refractory epilepsy (82% with partial seizures) in 23 different clinics in eight European countries.
2 This was an open multicentre study in which patients who had experienced a significant benefit from vigabatrin and had continued to take the drug for 1 year or longer were eligible for evaluation. The mean duration of therapy in the 254 patients was 22.7 months; 72 patients received vigabatrin for more than 2 years.
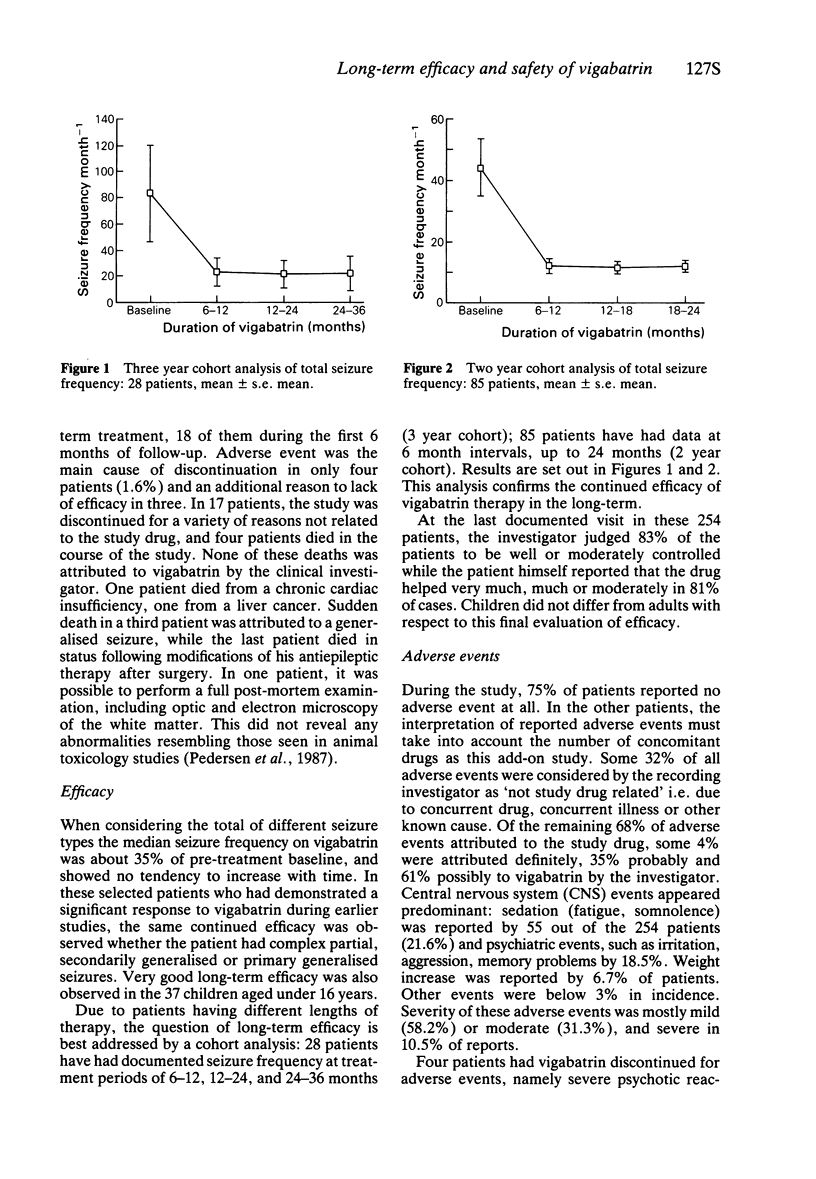
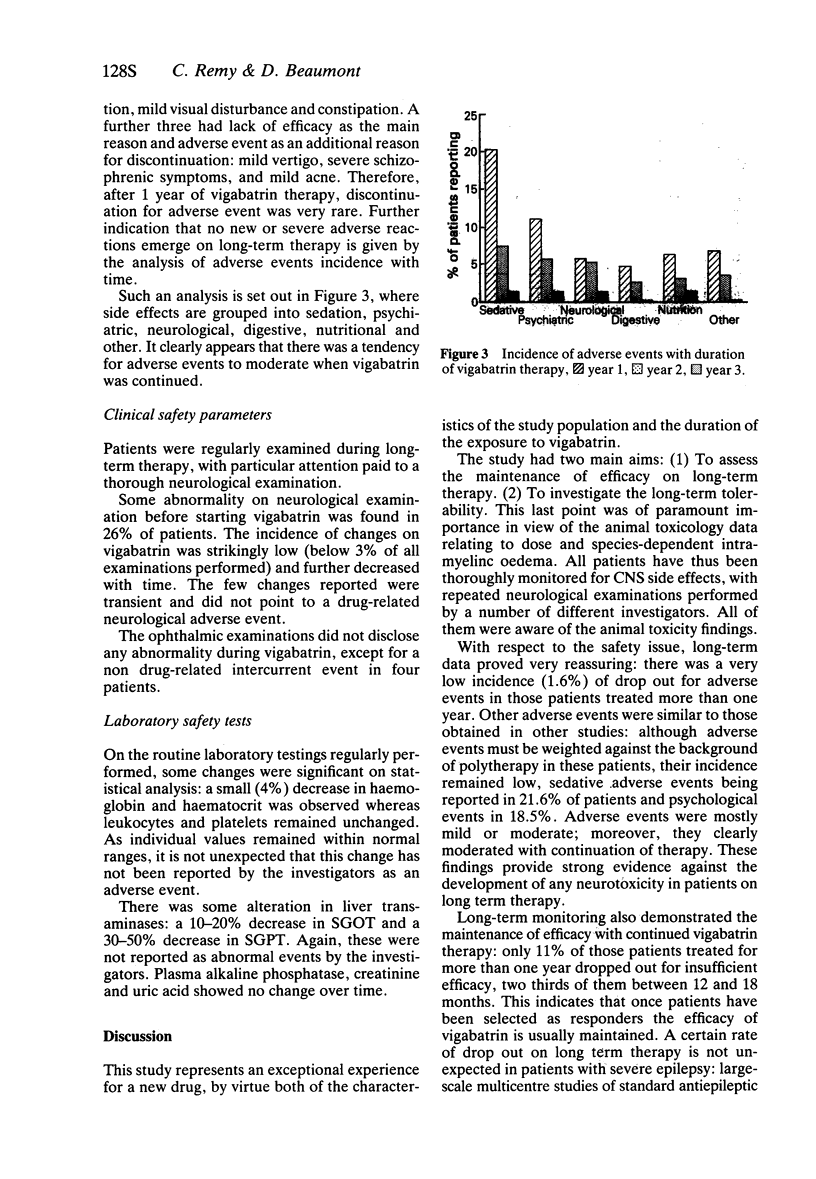
3 Patients were severely affected by epilepsy with a median monthly seizure frequency of 15.7 despite taking an average of 2.2 antiepileptic drugs. On vigabatrin, the median seizure frequency was about 35% of baseline, remaining stable over time despite a 10% reduction in the number of concurrent medications.
4 The lack of tachyphylaxis to the antiepileptic effect of vigabatrin is shown by the small number of patients who discontinued for insufficient efficacy (11%), two thirds of them during the first 6 months of follow-up. Maintenance of efficacy is also clearly demonstrated by analysis of 2 year and 3 year cohorts of patients.
5 Clinical and biological tolerability was excellent. There was a very low rate of drop out for adverse events (1.6%). Adverse events, mainly sedation, irritation and weight gain were mostly mild and transient. 75% of patients reported no adverse event at all.
6 Safety evaluation included serial neurological, ophthalmological and general examinations: no new abnormal clinical feature or adverse event emerged with long term therapy.
Keywords: vigabatrin, efficacy, safety, refractory epilepsy
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. H., Ford G. P., Newberne J. W. A study of the effects of vigabatrin on the central nervous system and retina of Sprague Dawley and Lister-Hooded rats. Toxicol Pathol. 1987;15(2):143–148. doi: 10.1177/019262338701500203. [DOI] [PubMed] [Google Scholar]
- Cosi V., Callieco R., Galimberti C. A., Manni R., Tartara A., Lanzi G., Balottin U., Perucca E. Effect of vigabatrin (gamma-vinyl-GABA) on visual, brainstem auditory and somatosensory evoked potentials in epileptic patients. Eur Neurol. 1988;28(1):42–46. doi: 10.1159/000116227. [DOI] [PubMed] [Google Scholar]
- Liegeois-Chauvel C., Marquis P., Gisselbrecht D., Pantieri R., Beaumont D., Chauvel P. Effects of long term vigabatrin on somatosensory evoked potentials in epileptic patients. Br J Clin Pharmacol. 1989;27 (Suppl 1):69S–72S. doi: 10.1111/j.1365-2125.1989.tb03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford J. P., Dam M. Meta-analysis of European placebo controlled studies of vigabatrin in drug resistant epilepsy. Br J Clin Pharmacol. 1989;27 (Suppl 1):101S–107S. doi: 10.1111/j.1365-2125.1989.tb03469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B., Højgaard K., Dam M. Vigabatrin: no microvacuoles in a human brain. Epilepsy Res. 1987 Jan;1(1):74–76. doi: 10.1016/0920-1211(87)90054-4. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Mattson R. H., Cramer J. A., Collins J. F., Novelly R. A., Craft B. Results of a nationwide Veterans Administration Cooperative Study comparing the efficacy and toxicity of carbamazepine, phenobarbital, phenytoin, and primidone. Epilepsia. 1987;28 (Suppl 3):S50–S58. doi: 10.1111/j.1528-1157.1987.tb05778.x. [DOI] [PubMed] [Google Scholar]


