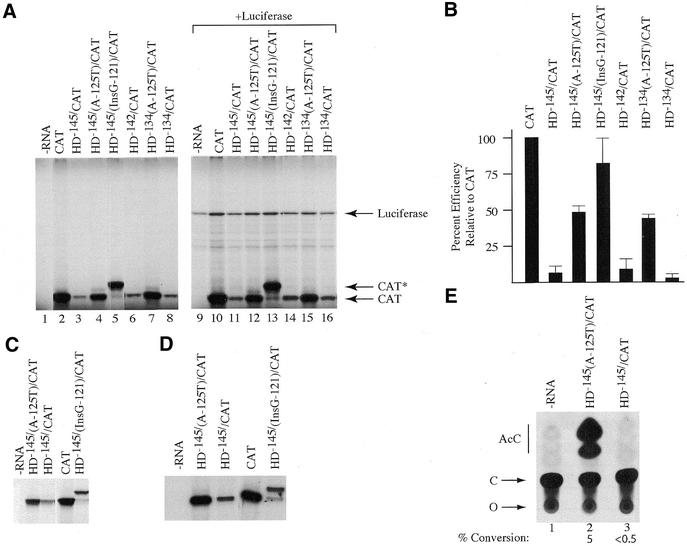
Figure 4.
In vitro and in vivo translation of HD expression constructs. (A) Translation of HD/CAT reporter constructs in wheat germ extracts. Following in vitro translations, samples were treated in SDS sample buffer and electrophoresed into a 12% SDS–polyacrylamide gel. The gel was treated with EN3Hance, dried and exposed to X-Omat (Kodak) film. The mRNA species used to program the lysate are indicated above the panel. The position of migration of Luciferase, CAT* and CAT protein is indicated to the right of the panel. Lanes 1 and 9, control translation performed without endogenously added mRNA template; lanes 2–8, translation of CAT and HD/CAT mRNAs; lane 10–16, co-translation of luciferase mRNA and either CAT or HD/CAT mRNAs. (B) Bar graph representing the average translation efficiencies of HD/CAT reporters. TCA precipitation of translated products was used to quantitate levels of protein product. Values are standardized to the efficiency obtained with CAT mRNA. All values represent the average of at least four experiments and include the experiment presented in (A). Standard deviations are presented. (C) Translation of HD/CAT reporter constructs in rabbit reticulocyte lysates. Following in vitro translations, samples were treated in SDS sample buffer and electrophoresed into a 12% SDS–polyacrylamide gel. The gel was treated with EN3Hance, dried and exposed to X-Omat (Kodak) film. The mRNA species used to program the lysate are indicated above the panel. (D) Translation of HD/CAT reporter constructs in Krebs extracts. The mRNA species used to program the lysate are indicated above the panel. (E) In vivo translation in X.laevis oocytes. An autoradiograph of a representative TLC for the CAT assays performed is shown. The percent conversion shown below the panel is the average obtained from four independent experiments. AcC, acetylated forms of chloramphenicol; C, chloramphenicol; O, origin.