Abstract
1. The accuracy of a computerised method of pharmacokinetic interpretation of a single serum theophylline concentration, employing the statistical technique of Bayesian analysis, has been evaluated for an oral slow release form of theophylline using twice daily dosing. 2. Twenty-four hour steady state serum theophylline concentration-time profiles of one Uniphyllin Continus 400 mg tablet (Napp Laboratories) every 12 h were measured in 15 patients. These profiles demonstrated a diurnal variation of theophylline absorption which was faster during the day. 3. Revised predictions of the profiles were generated by Bayesian analysis using a single serum theophylline concentration taken during a previous outpatient appointment. Comparing the predicted and measured profiles, the accuracy of the Bayesian method is considered more than adequate for clinical purposes. 4. The predictions produced by the revised estimates were statistically less biased and more precise than those derived by a theophylline algorithm using population data. 5. The mean prediction errors of the revised estimates of the day and night-peak drug concentrations were -0.55 mg l-1 and -0.21 mg l-1 whilst those of the evening and morning troughs were 1.17 mg l-1 and 0.41 mg l-1, respectively. 6. Analysis of the predictive and relative performance of the samples drawn during the profile revealed that the sample taken prior to a morning dose produced the most accurate predictions. 7. There was no statistical difference in the relative predictive performance of samples drawn up to 4 h before or 2 h after the morning dose. It is, therefore, recommended that all serum theophylline concentrations to be used in Bayesian analysis, should be drawn within this period.
Full text
PDF


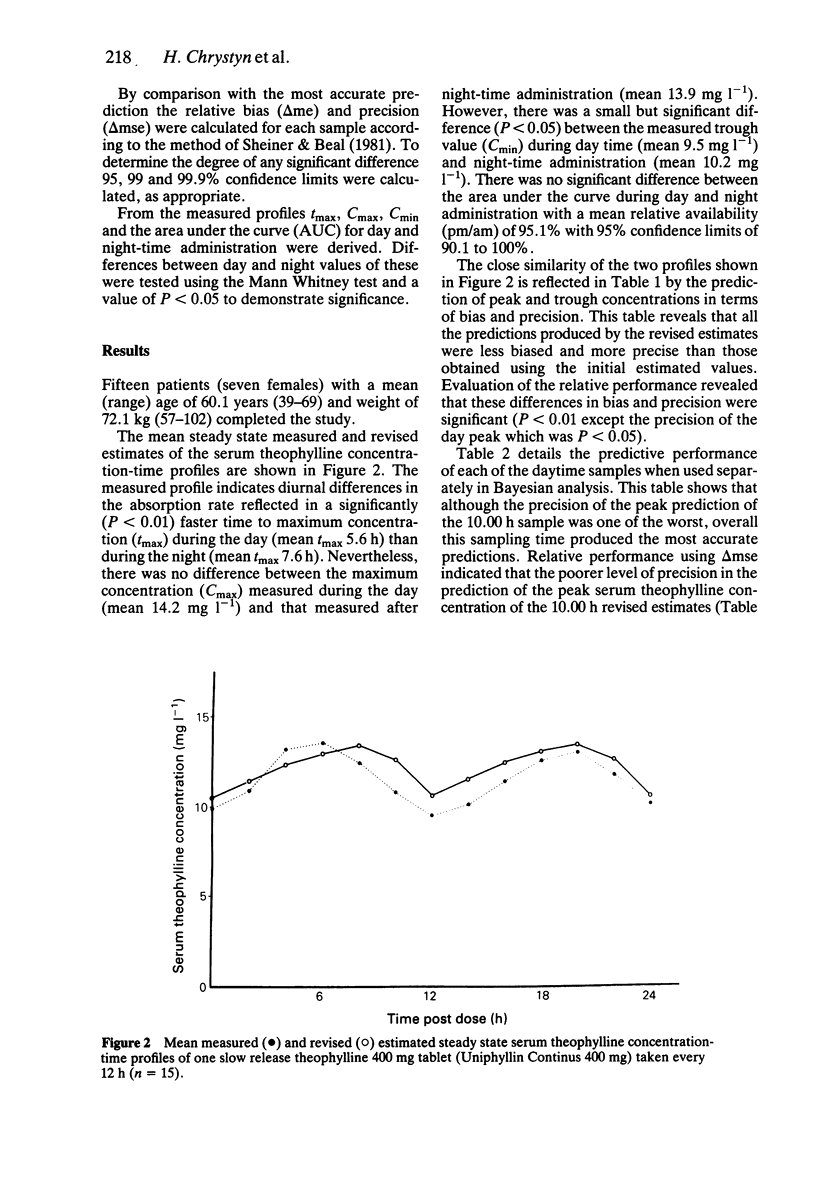
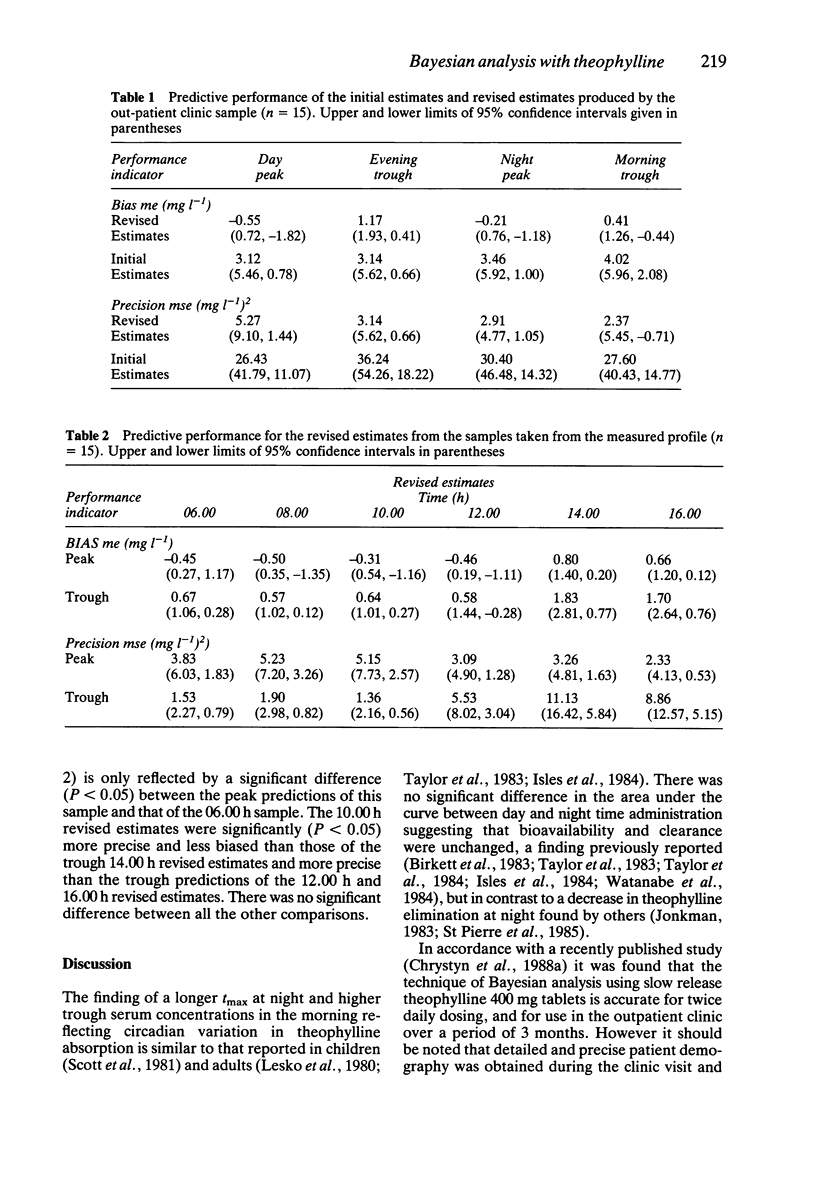


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrystyn H., Ellis J. W., Mulley B. A., Peake M. D. The accuracy and stability of Bayesian theophylline predictions. Ther Drug Monit. 1988;10(3):299–305. doi: 10.1097/00007691-198803000-00011. [DOI] [PubMed] [Google Scholar]
- Chrystyn H., Mulley B. A., Peake M. D. The accuracy of a pharmacokinetic theophylline predictor using once daily dosing. Br J Clin Pharmacol. 1987 Sep;24(3):301–307. doi: 10.1111/j.1365-2125.1987.tb03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. C. Audit of serum theophylline concentrations in patients from general practice. J R Coll Gen Pract. 1987 Mar;37(296):105–106. [PMC free article] [PubMed] [Google Scholar]
- Jenne J. W., Wyze M. S., Rood F. S., MacDonald F. M. Pharmacokinetics of theophylline. Application to adjustment of the clinical dose of aminophylline. Clin Pharmacol Ther. 1972 May-Jun;13(3):349–360. doi: 10.1002/cpt1972133349. [DOI] [PubMed] [Google Scholar]
- Jonkman J. H., van der Boon W. J. Nocturnal theophylline plasma concentrations. Lancet. 1983 Jun 4;1(8336):1278–1279. doi: 10.1016/s0140-6736(83)92728-9. [DOI] [PubMed] [Google Scholar]
- Lesko L. J., Brousseau D., Canada A. T., Eastwood G. Temporal variations in trough serum theophylline concentrations at steady state. J Pharm Sci. 1980 Mar;69(3):358–359. doi: 10.1002/jps.2600690332. [DOI] [PubMed] [Google Scholar]
- Scott P. H., Tabachnik E., MacLeod S., Correia J., Newth C., Levison H. Sustained-release theophylline for childhood asthma: evidence for circadian variation of theophylline pharmacokinetics. J Pediatr. 1981 Sep;99(3):476–479. doi: 10.1016/s0022-3476(81)80354-x. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B., Beal S. L. Bayesian individualization of pharmacokinetics: simple implementation and comparison with non-Bayesian methods. J Pharm Sci. 1982 Dec;71(12):1344–1348. doi: 10.1002/jps.2600711209. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B., Beal S. L. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981 Aug;9(4):503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- St-Pierre M. V., Spino M., Isles A. F., Tesoro A., MacLeod S. M. Temporal variation in the disposition of theophylline and its metabolites. Clin Pharmacol Ther. 1985 Jul;38(1):89–95. doi: 10.1038/clpt.1985.140. [DOI] [PubMed] [Google Scholar]
- Taylor D. R., Duffin D., Kinney C. D., McDevitt D. G. Circadian variation in plasma theophylline concentrations during maintenance therapy with a sustained-release preparation in patients with obstructive airways disease. Br J Clin Pharmacol. 1984 Jul;18(1):27–30. doi: 10.1111/j.1365-2125.1984.tb05017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. R., Duffin D., Kinney C. D., McDevitt D. G. Investigation of diurnal changes in the disposition of theophylline. Br J Clin Pharmacol. 1983 Oct;16(4):413–416. doi: 10.1111/j.1365-2125.1983.tb02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Nakano S., Nagai K., Ogawa N. Time-dependent absorption of theophylline in man. J Clin Pharmacol. 1984 Nov-Dec;24(11-12):509–514. doi: 10.1002/j.1552-4604.1984.tb02760.x. [DOI] [PubMed] [Google Scholar]


