Figure 7.
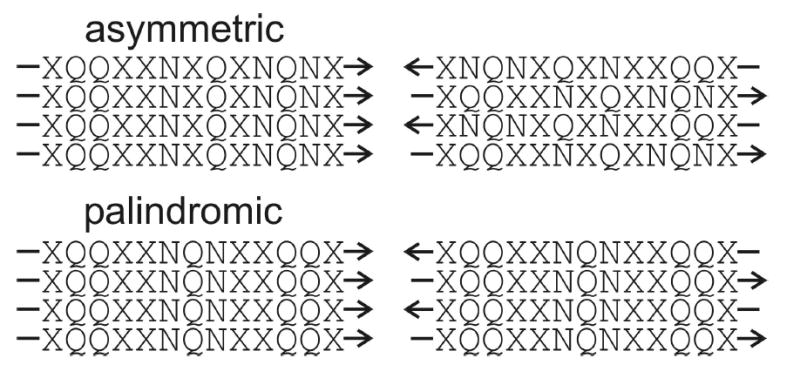
Proposal regarding the preference for in-register parallel (left) or antiparallel (right) β-sheets in amyloid fibrils formed by peptides with glutamine- and asparagine-rich sequences. Sequences with asymmetric distributions of glutamine and asparagine residues (such as Ure2p10–39) can maximize their polar zipper interactions only in an in-register parallel β-sheet, making this the preferred structure. Sequences with palindromic distributions of glutamine and asparagine residues can maximize their polar zipper interactions in either type of β-sheet, allowing electrostatic or other interactions to determine the preferred structure.
