Abstract
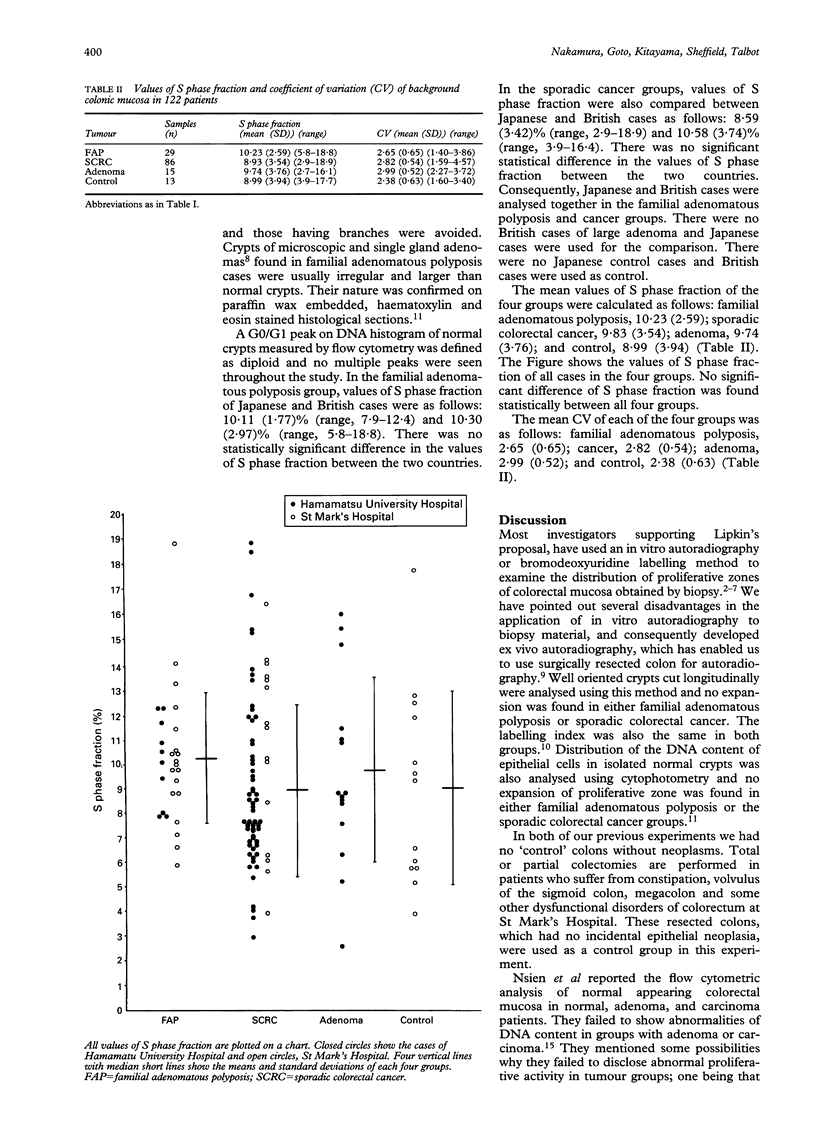
DNA synthetic (S) phase fractions of normal appearing colonic mucosa in Japanese and British patients with colorectal neoplasms were compared with those in patients without colonic neoplasms. Normal crypts were isolated from fresh surgical specimens of the large intestine by the use of EDTA. After fixation with 70% ethanol, isolated crypts were digested with pepsin into single nuclei suspensions. These were stained with propidium iodide and examined by flow cytometry. S phase fraction was calculated from the flow cytometry DNA histogram using Baisch's method. S phase fractions of normal appearing crypts in Japanese and British patients with colorectal tumours were not significantly different and analysed together. S phase fraction of normal appearing colonic crypts in 14 patients with familial adenomatous polyposis (FAP) was 10.23 (2.59%) (mean SD)) ranging from 5.8 to 18.8. S phase fraction of background normal mucosal in patients with large adenomas (over 2 cm) and adenocarcinomas were 9.74 (3.76%) (range, 2.7-16.1) and 8.93 (3.54%) (range, 2.9-18.9) respectively. In normal mucosa of patients without any colorectal neoplasms, S phase fraction was 8.99 (3.94)% (range, 3.9-17.7). There was no statistically significant difference in S phase fractions of normal mucosa in the four groups. Our results show that an increase in proliferative activity of background colonic crypts is not necessary for tumour development.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Kino I. Morphometrical and cell kinetic studies of normal human colorectal mucosa. Comparison between the proximal and the distal large intestine. Acta Pathol Jpn. 1989 Nov;39(11):725–730. doi: 10.1111/j.1440-1827.1989.tb02421.x. [DOI] [PubMed] [Google Scholar]
- Baisch H., Göhde W., Linden W. A. Analysis of PCP-data to determine the fraction of cells in the various phases of cell cycle. Radiat Environ Biophys. 1975 Jun 13;12(1):31–39. doi: 10.1007/BF02339807. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Lipkin M. Proliferative patterns in colonic mucosa in familial polyposis. Cancer. 1975 Feb;35(2):413–418. doi: 10.1002/1097-0142(197502)35:2<413::aid-cncr2820350217>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Galloway D. J., Jarrett F., Boyle P., Indran M., Carr K., Owen R. W., George W. D. Morphological and cell kinetic effects of dietary manipulation during colorectal carcinogenesis. Gut. 1987 Jun;28(6):754–763. doi: 10.1136/gut.28.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama T., Utzunomiya J., Sasaki J. Epithelial cell kinetics in the crypts of familial polyposis of colon. Jpn J Surg. 1977 Dec;7(4):230–234. doi: 10.1007/BF02469355. [DOI] [PubMed] [Google Scholar]
- Kouri M., Pyrhönen S., Mecklin J. P., Järvinen H., Laasonen A., Franssila K., Nordling S. The prognostic value of DNA-ploidy in colorectal carcinoma: a prospective study. Br J Cancer. 1990 Dec;62(6):976–981. doi: 10.1038/bjc.1990.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin M. Phase 1 and phase 2 proliferative lesions of colonic epithelial cells in diseases leading to colonic cancer. Cancer. 1974 Sep;34(3):suppl–suppl:888. doi: 10.1002/1097-0142(197409)34:3+<878::aid-cncr2820340715>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Goto J., Kitayama M., Kino I. Application of the crypt-isolation technique to flow-cytometric analysis of DNA content in colorectal neoplasms. Gastroenterology. 1994 Jan;106(1):100–107. doi: 10.1016/s0016-5085(94)94651-5. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Kino I., Baba S. Cell kinetics analysis of background colonic mucosa of patients with intestinal neoplasms by ex vivo autoradiography. Gut. 1988 Jul;29(7):997–1002. doi: 10.1136/gut.29.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Kino I., Baba S. Ex vivo autoradiography of the human gastrointestinal tract: a new approach to cell kinetic studies of surgically removed tumor-bearing organs. Gan. 1983 Feb;74(1):116–121. [PubMed] [Google Scholar]
- Nakamura S., Kino I., Baba S. Nuclear DNA content of isolated crypts of background colonic mucosa from patients with familial adenomatous polyposis and sporadic colorectal cancer. Gut. 1993 Sep;34(9):1240–1244. doi: 10.1136/gut.34.9.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Kino I. Morphogenesis of minute adenomas in familial polyposis coli. J Natl Cancer Inst. 1984 Jul;73(1):41–49. [PubMed] [Google Scholar]
- Nsien E., Steinberg W. M., Wilkinson D. S., Rhame J. G., Henry J. P. Single-parameter DNA flow cytometric analysis of normal-appearing colonic mucosa does not predict the presence of colonic neoplasia. Am J Gastroenterol. 1991 Oct;86(10):1477–1481. [PubMed] [Google Scholar]
- Paganelli G. M., Biasco G., Santucci R., Brandi G., Lalli A. A., Miglioli M., Barbara L. Rectal cell proliferation and colorectal cancer risk level in patients with nonfamilial adenomatous polyps of the large bowel. Cancer. 1991 Dec 1;68(11):2451–2454. doi: 10.1002/1097-0142(19911201)68:11<2451::aid-cncr2820681121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Kellett M., Rew D. A., Roberts S. A. Proliferation in human gastrointestinal epithelium using bromodeoxyuridine in vivo: data for different sites, proximity to a tumour, and polyposis coli. Gut. 1992 Apr;33(4):524–529. doi: 10.1136/gut.33.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risio M., Lipkin M., Candelaresi G., Bertone A., Coverlizza S., Rossini F. P. Correlations between rectal mucosa cell proliferation and the clinical and pathological features of nonfamilial neoplasia of the large intestine. Cancer Res. 1991 Apr 1;51(7):1917–1921. [PubMed] [Google Scholar]
- Roncucci L., Scalmati A., Ponz de Leon M. Pattern of cell kinetics in colorectal mucosa of patients with different types of adenomatous polyps of the large bowel. Cancer. 1991 Aug 15;68(4):873–878. doi: 10.1002/1097-0142(19910815)68:4<873::aid-cncr2820680433>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Murakami T. Clinical application of flow cytometry for DNA analysis of solid tumors. Acta Pathol Jpn. 1992 Jan;42(1):1–14. doi: 10.1111/j.1440-1827.1992.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Seckinger D., Sugarbaker E., Frankfurt O. DNA content in human cancer. Application in pathology and clinical medicine. Arch Pathol Lab Med. 1989 Jun;113(6):619–626. [PubMed] [Google Scholar]
- Wargovich M. J., Baer A. R., Hu P. J., Sumiyoshi H. Dietary factors and colorectal cancer. Gastroenterol Clin North Am. 1988 Dec;17(4):727–745. [PubMed] [Google Scholar]
- Witzig T. E., Loprinzi C. L., Gonchoroff N. J., Reiman H. M., Cha S. S., Wieand H. S., Katzmann J. A., Paulsen J. K., Moertel C. G. DNA ploidy and cell kinetic measurements as predictors of recurrence and survival in stages B2 and C colorectal adenocarcinoma. Cancer. 1991 Aug 15;68(4):879–888. doi: 10.1002/1097-0142(19910815)68:4<879::aid-cncr2820680434>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Yamada K., Yoshitake K., Sato M., Ahnen D. J. Proliferating cell nuclear antigen expression in normal, preneoplastic, and neoplastic colonic epithelium of the rat. Gastroenterology. 1992 Jul;103(1):160–167. doi: 10.1016/0016-5085(92)91109-h. [DOI] [PubMed] [Google Scholar]


