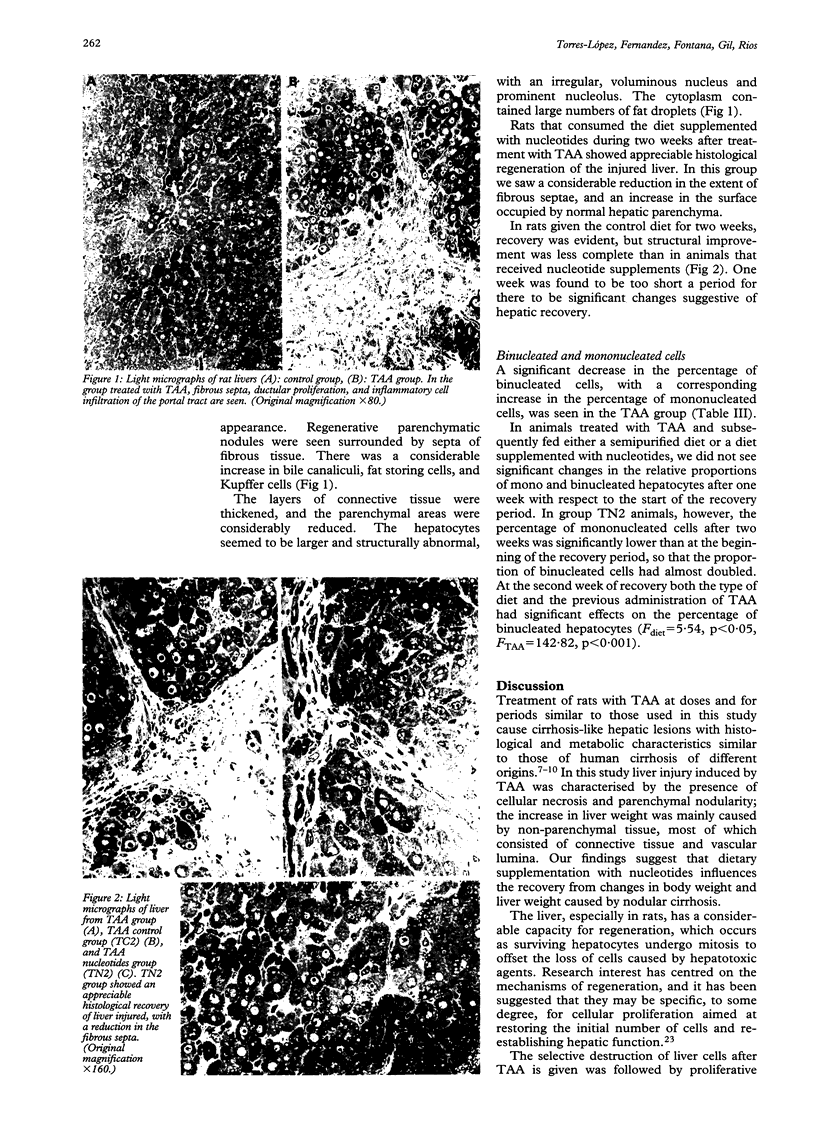
Abstract
Intake of thioacetamide in drinking water causes liver cirrhosis in rats, which exhibit many changes similar to human disease. Nucleotides play an important part in major cellular functions, and recent studies suggest that dietary nucleotides may be considered 'semi-essential' nutrients in situations when an inadequate dietary supply may affect the growth of tissues with a rapid turnover rate. The aim of this study was to assess the effect of dietary nucleotides on lesions in thioacetamide-cirrhotic rats, and to calculate the proportion of mono and binucleated hepatocytes in different experimental groups. Rats were given cirrhosis by oral intake of thioacetamide in the drinking water (300 mg/l) for four months. One group was treated with a standard nucleotide free diet, and another group was treated with the same diet supplemented with 250 mg of nucleotides per 100 g of diet for one and two weeks. A striking reduction (mean (SEM)) in the proportion of binucleated cells was seen in thioacetamide-cirrhotic rats (4.8 (1.3) v 21.4 (1.0)), showing a change in the mitotic mechanism in focal lesions. Cirrhotic rats that consumed a semipurified diet supplemented with nucleotides during two weeks showed considerable histological regeneration of the injured liver. These animals had significantly higher proportion of binucleated cells than did animals at the beginning of the recovery period (8.2 (1.2) v 4.8 (1.3)). In the second week of recovery, both types of diet (F = 5.54, p < 0.05) and the previous administration of thioacetamide (F = 142.82, p < 0.001) had significant effects on the percentage of binucleated hepatocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anghileri L. J., Heidbreder M., Weiler G., Dermietzel R. Hepatocarcinogenesis by thioacetamide: correlations of histological and biochemical changes, and possible role of cell injury. Exp Cell Biol. 1977;45(1-2):34–47. doi: 10.1159/000162856. [DOI] [PubMed] [Google Scholar]
- Becker F. F. Thioacetamide hepatocarcinogenesis. J Natl Cancer Inst. 1983 Sep;71(3):553–558. [PubMed] [Google Scholar]
- Dasgupta A., Chatterjee R., Chowdhury J. R. Thioacetamide-induced hepatocarcinoma in rat. Oncology. 1981;38(4):249–253. doi: 10.1159/000225560. [DOI] [PubMed] [Google Scholar]
- Díez-Fernández C., Boscá L., Fernández-Simón L., Alvarez A., Cascales M. Relationship between genomic DNA ploidy and parameters of liver damage during necrosis and regeneration induced by thioacetamide. Hepatology. 1993 Oct;18(4):912–918. doi: 10.1002/hep.1840180424. [DOI] [PubMed] [Google Scholar]
- GUPTA D. N. Production of cancer of the bile ducts with thioacetamide. Nature. 1955 Feb 5;175(4449):257–257. doi: 10.1038/175257a0. [DOI] [PubMed] [Google Scholar]
- Jack E. M., Bentley P., Bieri F., Muakkassah-Kelly S. F., Stäubli W., Suter J., Waechter F., Cruz-Orive L. M. Increase in hepatocyte and nuclear volume and decrease in the population of binucleated cells in preneoplastic foci of rat liver: a stereological study using the nucleator method. Hepatology. 1990 Feb;11(2):286–297. doi: 10.1002/hep.1840110220. [DOI] [PubMed] [Google Scholar]
- Kachi K., French S. W. The connection between the nuclei of binucleated hepatocytes: an ultrastructural study. J Submicrosc Cytol Pathol. 1994 Apr;26(2):163–172. [PubMed] [Google Scholar]
- Kretzschmar M., Machnik G., Müller A., Splinter F. K., Zimmermann T., Klinger W. Experimental treatment of thioacetamide-induced liver cirrhosis by metenolone acetate. A morphological and biochemical study. Exp Pathol. 1991;42(1):37–46. doi: 10.1016/s0232-1513(11)80036-8. [DOI] [PubMed] [Google Scholar]
- Latifi R., Killam R. W., Dudrick S. J. Nutritional support in liver failure. Surg Clin North Am. 1991 Jun;71(3):567–578. doi: 10.1016/s0039-6109(16)45434-4. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Mullen K. D., McCullough A. J. Problems with animal models of chronic liver disease: suggestions for improvement in standardization. Hepatology. 1989 Mar;9(3):500–503. doi: 10.1002/hep.1840090326. [DOI] [PubMed] [Google Scholar]
- Müller D., Sommer M., Kretzschmar M., Zimmermann T., Buko V. U., Lukivskaya O., Dargel R. Lipid peroxidation in thioacetamide-induced macronodular rat liver cirrhosis. Arch Toxicol. 1991;65(3):199–203. doi: 10.1007/BF02307309. [DOI] [PubMed] [Google Scholar]
- Müller M. J., Rieger A., Willmann O., Lautz H. U., Balks H. J., Von Zur Mühlen A., Canzler H., Schmidt F. W. Metabolic responses to lipid infusions in patients with liver cirrhosis. Clin Nutr. 1992 Aug;11(4):193–206. doi: 10.1016/0261-5614(92)90028-o. [DOI] [PubMed] [Google Scholar]
- Ogoshi S., Iwasa M., Kitagawa S., Ohmori Y., Mizobuchi S., Iwasa Y., Tamiya T. Effects of total parenteral nutrition with nucleoside and nucleotide mixture on D-galactosamine-induced liver injury in rats. JPEN J Parenter Enteral Nutr. 1988 Jan-Feb;12(1):53–57. doi: 10.1177/014860718801200153. [DOI] [PubMed] [Google Scholar]
- Ogoshi S., Iwasa M., Yonezawa T., Tamiya T. Effect of nucleotide and nucleoside mixture on rats given total parenteral nutrition after 70% hepatectomy. JPEN J Parenter Enteral Nutr. 1985 May-Jun;9(3):339–342. doi: 10.1177/0148607185009003339. [DOI] [PubMed] [Google Scholar]
- Ohyanagi H., Nishimatsu S., Kanbara Y., Usami M., Saitoh Y. Effects of nucleosides and a nucleotide on DNA and RNA syntheses by the salvage and de novo pathway in primary monolayer cultures of hepatocytes and hepatoma cells. JPEN J Parenter Enteral Nutr. 1989 Jan-Feb;13(1):51–58. doi: 10.1177/014860718901300151. [DOI] [PubMed] [Google Scholar]
- Palombo J. D., Bowers J. L., Clouse M. E., McCullough A., Forse R. A., Bistrian B. R. Hepatic utilization of exogenous nucleotide precursors for restoration of ATP after cold ischemia in rats. Am J Clin Nutr. 1993 Mar;57(3):420–427. doi: 10.1093/ajcn/57.3.420. [DOI] [PubMed] [Google Scholar]
- Popper H. Pathologic aspects of cirrhosis. A review. Am J Pathol. 1977 Apr;87(1):228–264. [PMC free article] [PubMed] [Google Scholar]
- Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983 Jan-Feb;3(1):112–120. doi: 10.1002/hep.1840030118. [DOI] [PubMed] [Google Scholar]
- Zimmermann T., Müller A., Machnik G., Franke H., Schubert H., Dargel R. Biochemical and morphological studies on production and regression of experimental liver cirrhosis induced by thioacetamide in Uje: WIST rats. Z Versuchstierkd. 1987;30(5-6):165–180. [PubMed] [Google Scholar]