Figure 2.
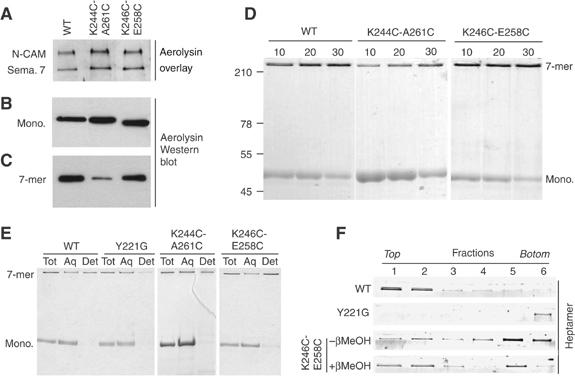
Receptor binding, heptamerization and membrane association of the disulfide-containing mutants. (A) The disulfide-containing mutants were tested in an aerolysin overlay assay on BHK cell extracts (40 μg protein per lane). Aerolysin was revealed using anti-aerolysin antibodies followed by an HRP-labeled secondary antibody. Binding to two GPI-anchored proteins, N-CAM and semaphorin 7 (sema. 7), is illustrated. (B) BHK cells were incubated with trypsin-activated WT or disulfide-containing mutant aerolysins (400 ng/ml) at 4°C for 1 h. Cell extracts (40 μg per lane), from which the nuclei were cleared by centrifugation, were analyzed by Western blotting using an anti-toxin antibody. (C) BHK cells, treated with trypsin-activated WT or mutant aerolysin as in panel B, were washed and further incubated at 37°C for 45 min. The presence of heptamers was revealed by Western blotting against aerolysin. (D) The ability of the disulfide-containing mutants to oligomerize in vitro was assayed by monitoring the appearance of the heptamer as a function of time after trypsin activation. SDS gels were revealed by Coomassie blue staining. (E) WT, Y221G and disulfide-containing mutant proaerolysins were activated with trypsin and allowed to heptamerize in vitro and then submitted to a Triton X-114 partitioning assay. Aliquots from the total sample (Tot), the aqueous (Aq) and detergent (Det) phases were analyzed by SDS–PAGE and Coomassie blue staining. (F) WT, Y221G, K246C-E258C and reduced K246C-E258C heptamer were reconstituted into proteoliposomes and submitted to separation by sucrose density flotation gradients. The six fractions of the gradient, from top to bottom, were analyzed by SDS–PAGE and Coomassie blue staining.
