Figure 7.
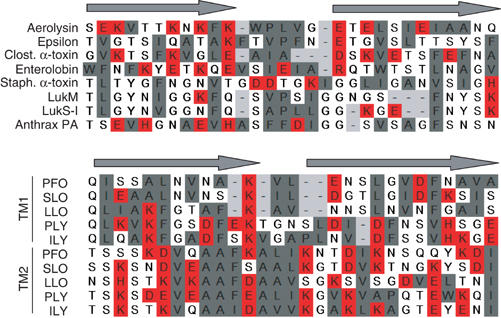
Alignments of putative transmembrane domains of different β-barrel PFT. The DIII-loop of aerolysin was aligned manually with the corresponding regions in C. perfringens epsilon-toxin (a toxin with a similar three-dimensional structure to aerolysin) (Cole et al, 2004), Clostridial α-toxin (Clost. α-toxin) and enterolobin, three Staphylococcal PFT (α-toxin, LukM, LukS-I) and the protective antigen (PA) of anthrax (Nassi et al, 2002) as well as the two transmembrane domains (TM1 and TM2) of five cholesterol-dependent cytolysins (PFO: perfringolysin; SLO: streptolysin; LLO: listeriolysin; PLY: pneumolysin; ILY: intermedilysin; Tweten et al, 2001). Alignments were generated based on the alternating pattern of polar and hydrophobic residues. Hydrophobic residues are shown in gray, charged residues in red and other residues are left uncolored.
