Table 1.
Carboamination reactions catalyzed by imidozirconocenes 14. [a]
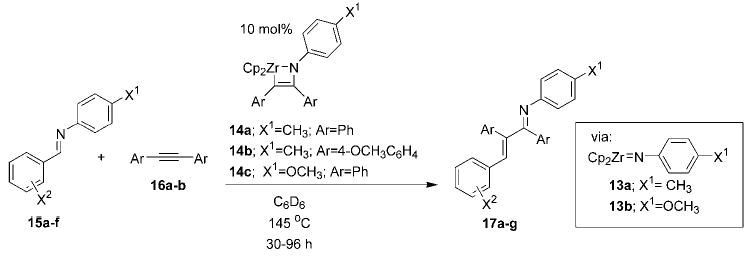
| Entry | Starting Imine | Alkyne | Catalyst | Product | Yield [%][b] |
|---|---|---|---|---|---|
| 1 | 15 a; X1 =CH3; X2 =4-CH3 | 16 a; Ar =Ph | 14 a | 17 a | 71 |
| 2 | 15 b; X1 =CH3; X2 =4-OCH3 | 16 a; Ar =Ph | 14 a | 17 b | 71 |
| 3 | 15 c; X1 =CH3; X2 =4-N(CH3)2 | 16 a; Ar =Ph | 14 a | 17 c | 82 |
| 4 | 15 d; X1 =OCH3; X2 =H | 16 a; Ar =Ph | 14 c | 17 d | 80 |
| 5 | 15 e; X1 =OCH3; X2 =4-CH3 | 16 a; Ar =Ph | 14 c | 17 e | 80 |
| 6 | 15 f; X1 =OCH3; X2 =3-CH3 | 16 a; Ar =Ph | 14 c | 17 f | 85 |
| 7[c] | 15 a; X1 =CH3; X2 =4-CH3 | 16 b; Ar =4-OCH3C6H4 | 14 b | 17 g | 58 |
Reaction is conducted with 10 mol% azazirconacyclobutene 14 (with appropriate X1 and Ar substitution) as catalyst and imine 15 and alkyne 16 at 0.5m in C6D6.
Yield of isolated product after chromatography.
Reaction was conducted at 160°C using a 3:1 mixture of 14 c:16 b.
