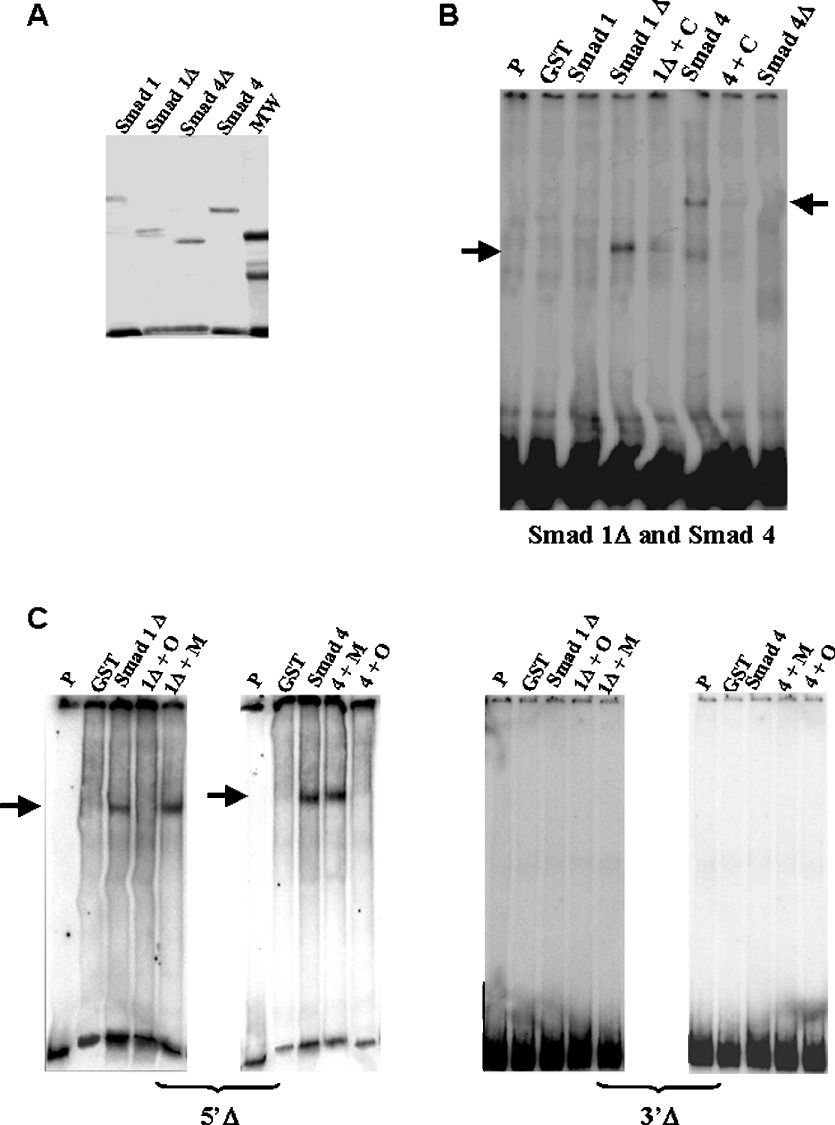
Figure 4. Purified SMAD 1 and SMAD 4 bind to BP and 5′Δ and 3′Δ fragments.
(A) Gel of purified recombinant proteins SMAD 1, SMAD 1Δ, SMAD 4 and SMAD 4Δ. MW, molecular mass standards. (B) Gel shift analysis of complexes between GST–SMAD fusion proteins and BP fragment. The protein used is indicated above each lane. Lanes indicated as ‘P’ and ‘GST’ show migration of the probe alone and reaction using GST peptide respectively. We observed binding reactions involving SMAD 1ΔMH2 (called SMAD 1Δ) and SMAD 4 (arrows). Competition reactions are indicated by the symbol ‘+C’ and correspond to addition of 100 times unlabelled BP. (C) Gel shift analysis of purified GST–SMAD 1ΔMH2 and SMAD 4 fusion protein complexes with the 5′Δ and 3′Δ fragments. The fragment used as a probe is indicated below each gel. Lanes indicated as ‘P’ and ‘GST’ show migration of the probe alone and reaction using GST peptide respectively. Competition reactions with 100 times unlabelled wild-type oligonucleotide for the SMAD consensus site are indicated as ‘+O’ and competition reactions with 100 times unlabelled mutated oligonucleotide for the SMAD consensus site are indicated as ‘+M’. The arrows show the specific binding.